Hydrogen Peroxide Generation and Hydrogen Oxidation Reaction on Pt/Co/Pt(111) and Pt/Co/Pt(100) Single-Crystal Model Catalyst Surface
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
To mitigate proton-exchange membrane (PEM) degradation, suppressing hydrogen peroxide (H2O2) generation is desired for the anode catalyst of PEM fuel cells (PEMFCs), while keeping the hydrogen oxidation reaction (HOR) activity. In this study, Pt/Co/Pt(111) and Pt/Co/Pt(100), approximately 2 nm-thick epitaxially stacked layers of Pt and Pt–Co alloy deposited on Pt(111) and Pt(100) single-crystal surfaces, respectively, were used as microstructural surface models of the Pt–Co anode catalyst, and the H2O2 generation and HOR mechanisms were discussed using the substrate generation/tip collection and tip generation/substrate collection modes of a scanning electrochemical microscope. We found that H2O2 generation on Pt/Co/Pt(111) was much lower than that on clean Pt(111), whereas the H2O2 generation property of Pt/Co/Pt(100) was similar to that of clean Pt(100). The influence of the underlying Co (Pt–Co) layers on H2O2 generation is discussed from the viewpoints of two previously proposed mechanisms: the adsorbed hydrogen (Hads)-related and water-adlayer-related mechanisms. Considering the applied potential dependence of H2O2 generation, the former Hads-related mechanism could not explain the H2O2 generation behavior of Pt/Co/Pt(100), whereas the latter water-adlayer-related mechanism could apply to both Pt/Co/Pt(111) and Pt/Co/Pt(100). Regarding the HOR, Pt/Co/Pt(100) showed a higher activity than that of clean Pt(100), whereas the activity of Pt/Co/Pt(111) was lower than that of the corresponding clean Pt(111). Such surface-atomic-arrangement-dependent HOR activities of Pt induced by the underlaid Co (Pt–Co) layers can be explained by weakened hydrogen adsorption energy, which can be rationalized by cyclic voltammogram features. The results clarify the alloying effect of Pt with Co for suppressing H2O2 generation while maintaining substantial HOR activity.
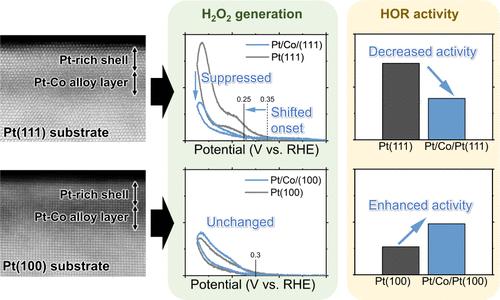
Pt/Co/Pt(111) 和 Pt/Co/Pt(100) 单晶模型催化剂表面的过氧化氢生成和氢氧化反应
为了减轻质子交换膜(PEM)的降解,PEM 燃料电池(PEMFCs)的阳极催化剂需要抑制过氧化氢(H2O2)的生成,同时保持氢氧化反应(HOR)的活性。本研究以分别沉积在铂(111)和铂(100)单晶表面的约 2 纳米厚的铂和铂钴合金外延堆积层 Pt/Co/Pt(111) 和 Pt/Co/Pt(100)作为铂钴阳极催化剂的微结构表面模型,并利用扫描电化学显微镜的基底生成/尖端收集和尖端生成/基底收集模式讨论了 H2O2 生成和氢氧化反应机理。我们发现,Pt/Co/Pt(111) 上的 H2O2 生成量远低于清洁 Pt(111),而 Pt/Co/Pt(100) 的 H2O2 生成特性与清洁 Pt(100) 相似。我们从之前提出的两种机制:吸附氢(Hads)相关机制和水层相关机制的角度讨论了底层 Co(Pt-Co)层对 H2O2 生成的影响。考虑到 H2O2 生成的外加电位依赖性,前一种与氢有关的机制无法解释 Pt/Co/Pt(100) 的 H2O2 生成行为,而后一种与水层有关的机制则适用于 Pt/Co/Pt(111) 和 Pt/Co/Pt(100)。在氢活性方面,Pt/Co/Pt(100)的活性高于洁净的 Pt(100),而 Pt/Co/Pt(111)的活性则低于相应的洁净 Pt(111)。下覆 Co(Pt-Co)层诱导的铂表面原子排列依赖性氢氧活性可以用氢吸附能的减弱来解释,而循环伏安图特征则可以说明这一点。这些结果阐明了铂与钴的合金化效应,即在抑制 H2O2 生成的同时保持大量的 HOR 活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


