Recyclable terthiophenes for synthesizing precision conjugated oligomers†
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
Precision polymers and oligomers are distinguished by their well-defined characteristics (e.g., chain length, monomer sequence). As a result, they have been leveraged in recent years to study numerous structure–property relationships ranging from photophysical characteristics to antimicrobial activity. However, the synthesis of precision polymers and oligomers often requires elaborate building blocks that are compatible with specific polymerization approaches. In most cases, these building blocks are neither recovered nor recycled, limiting the efficiency and sustainability of these synthetic methods. Herein, we report the first synthesis of a thiophene-bearing nickel(ii) external initiator that is used to synthesize modular and regioregular terthiophene. We then show that the halogenated terthiophene is a compatible building block in synthesizing precision oligothiophenes. This thiophene-bearing external initiator exhibits remarkable reactivity and stability through polymerization. Furthermore, we show that excess halogenated terthiophene can be recovered post-oligomerization and reused in subsequent syntheses. Overall, this work highlights the feasibility of synthesizing all-thiophene polymers using thiophene-bearing external initiators and recycling the modular terthiophene after oligomerization.
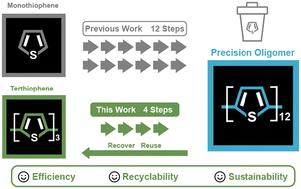
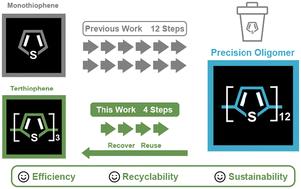
用于合成精密共轭低聚物的可回收噻吩类化合物
精密聚合物和低聚物具有明确的特征(如链长、单体序列)。因此,近年来人们利用它们来研究从光物理性质到抗菌活性等多种结构-性质关系。然而,合成精密聚合物和低聚物通常需要与特定聚合方法相匹配的精细构筑模块。在大多数情况下,这些构筑模块既不能回收,也不能循环使用,从而限制了这些合成方法的效率和可持续性。在此,我们首次报道了含噻吩的镍(II)外部引发剂的合成,这种引发剂可用于合成模块化和多态的三噻吩。然后,我们证明卤代噻吩是合成精密低聚噻吩的兼容构件。这种含噻吩的外部引发剂在聚合过程中表现出显著的反应活性和稳定性。此外,我们还展示了过量卤代噻吩可在聚合后回收,并在后续合成中重复使用。总之,这项工作强调了使用含噻吩的外部引发剂合成全噻吩聚合物并在低聚后回收模块化噻吩的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


