Inverse blebs operate as hydraulic pumps during mouse blastocyst formation
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
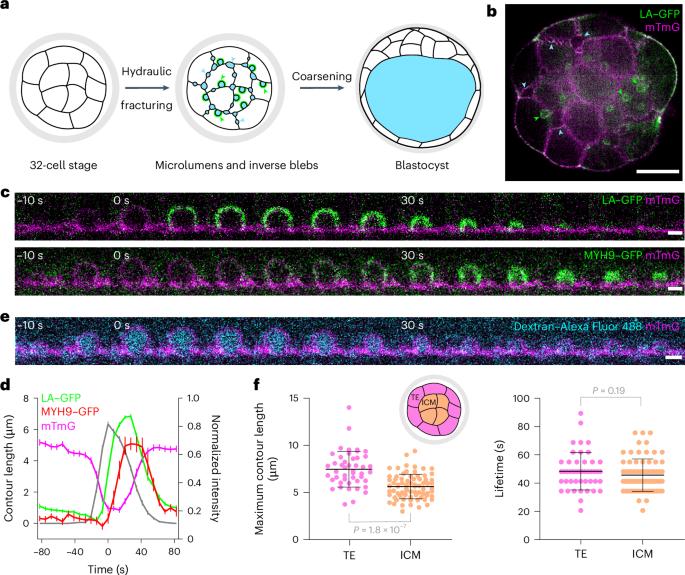
During preimplantation development, mouse embryos form a fluid-filled lumen. Pressurized fluid fractures cell–cell contacts and accumulates into pockets, which coarsen into a single lumen. How the embryo controls intercellular fluid movement during coarsening is unknown. Here we report inverse blebs growing into cells at adhesive contacts. Throughout the embryo we observed hundreds of inverse blebs, each filling with intercellular fluid and retracting within a minute. Inverse blebs grow due to pressure build-up resulting from fluid accumulation and cell–cell adhesion, which locally confines fluid. Inverse blebs retract due to actomyosin contraction, practically pushing fluid within the intercellular space. Importantly, inverse blebs occur infrequently at contacts formed by multiple cells, which effectively serve as fluid sinks. Manipulation of the embryo topology reveals that without sinks inverse blebs pump fluid into one another in futile cycles. We propose that inverse blebs operate as hydraulic pumps to promote luminal coarsening, thereby constituting an instrument used by cells to control fluid movement. Schliffka et al. show that in the early mouse embryo, hemispherical intrusions, or inverse blebs, grow into cells at cell–cell adhesion sites in response to luminal fluid accumulation and pressure build-up, and may serve as pumps moving fluid into hydraulic sinks.
在小鼠胚泡形成过程中,反向出血作为液压泵起作用
在胚胎植入前的发育过程中,小鼠胚胎会形成一个充满液体的管腔。加压的液体使细胞与细胞之间的接触断裂并积聚成袋,然后逐渐变粗成为一个单一的管腔。胚胎在粗化过程中如何控制细胞间液体运动尚不清楚。在这里,我们报告了在粘连接触处向细胞内生长的反向出血点。在整个胚胎中,我们观察到了数百个反向出血点,每个出血点都充满了细胞间液,并在一分钟内缩回。反向出血点的生长是由于液体积聚和细胞-细胞粘连导致的压力增加,从而在局部限制了液体。反向出血点由于肌动蛋白收缩而缩回,实际上是将液体推入细胞间隙。重要的是,反向出血点很少出现在由多个细胞形成的接触点上,这些接触点可有效充当液体汇。对胚胎拓扑结构的操作显示,如果没有汇,反向出血点就会在徒劳的循环中将液体泵入彼此。我们认为,反向出血点像液压泵一样促进管腔变粗,从而成为细胞控制液体运动的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


