Solid tumour-induced systemic immunosuppression involves dichotomous myeloid–B cell interactions
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
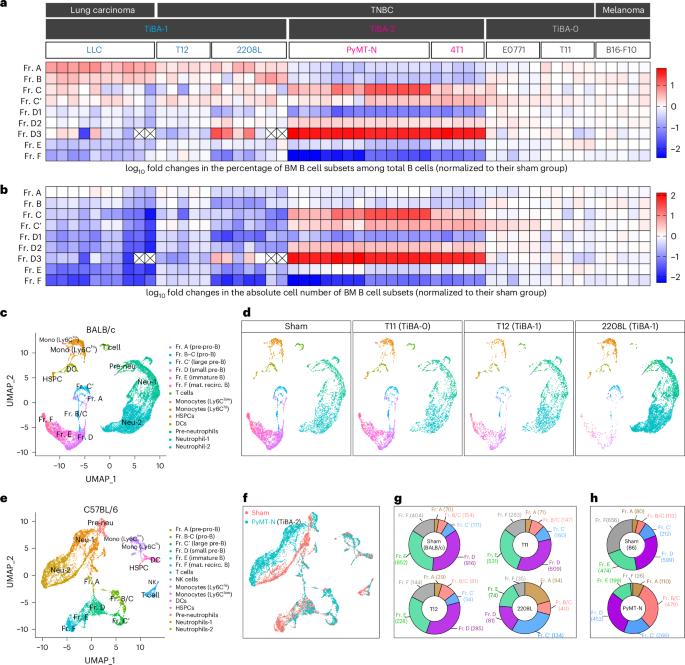
Solid tumours induce systemic immunosuppression that involves myeloid and T cells. B cell-related mechanisms remain relatively understudied. Here we discover two distinct patterns of tumour-induced B cell abnormality (TiBA; TiBA-1 and TiBA-2), both associated with abnormal myelopoiesis in the bone marrow. TiBA-1 probably results from the niche competition between pre-progenitor-B cells and myeloid progenitors, leading to a global reduction in downstream B cells. TiBA-2 is characterized by systemic accumulation of a unique early B cell population, driven by interaction with excessive neutrophils. Importantly, TiBA-2-associated early B cells foster the systemic accumulation of exhaustion-like T cells. Myeloid and B cells from the peripheral blood of patients with triple-negative breast cancer recapitulate the TiBA subtypes, and the distinct TiBA profile correlates with pathologic complete responses to standard-of-care immunotherapy. This study underscores the inter-patient diversity of tumour-induced systemic changes and emphasizes the need for treatments tailored to different B and myeloid cell abnormalities. Hao, Shen and colleagues identify and characterize two distinct types of myeloid–B cell interaction that may signal solid tumour-induced immunosuppression and can correlate with complete responses to immunotherapy in patients with breast cancer.

实体瘤诱导的全身免疫抑制涉及二分法髓系-B 细胞相互作用
实体瘤引起的全身免疫抑制涉及骨髓细胞和 T 细胞。与 B 细胞相关的机制研究相对较少。在这里,我们发现了两种不同的肿瘤诱导 B 细胞异常(TiBA;TiBA-1 和 TiBA-2)模式,这两种模式都与骨髓中的异常骨髓造血有关。TiBA-1 可能是由于前祖细胞-B 细胞和骨髓祖细胞之间的生态位竞争,导致下游 B 细胞全面减少。TiBA-2 的特点是,在与过多中性粒细胞相互作用的驱动下,一种独特的早期 B 细胞群在全身积聚。重要的是,与TiBA-2相关的早期B细胞会促进衰竭样T细胞的系统性积累。三阴性乳腺癌患者外周血中的骨髓细胞和B细胞再现了TiBA亚型,独特的TiBA特征与标准护理免疫疗法的病理完全反应相关。这项研究强调了肿瘤诱导的全身性变化在患者间的多样性,并强调了针对不同B细胞和骨髓细胞异常进行治疗的必要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


