DOR activation in mature oligodendrocytes regulates α-ketoglutarate metabolism leading to enhanced remyelination in aged mice
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
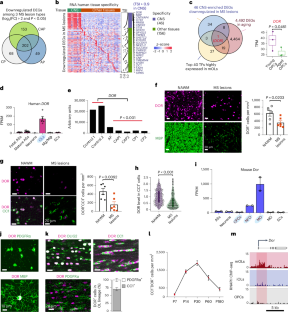
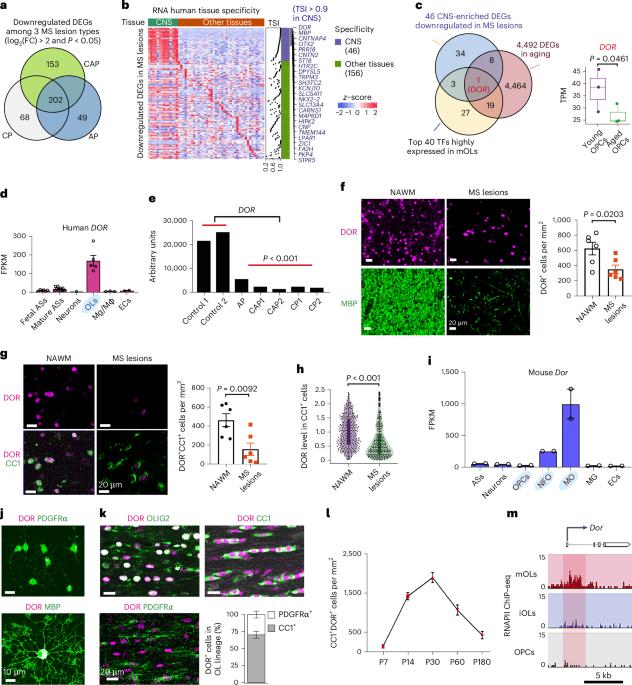
The decreased ability of mature oligodendrocytes to produce myelin negatively affects remyelination in demyelinating diseases and aging, but the underlying mechanisms are incompletely understood. In the present study, we identify a mature oligodendrocyte-enriched transcriptional coregulator diabetes- and obesity-related gene (DOR)/tumor protein p53-inducible nuclear protein 2 (TP53INP2), downregulated in demyelinated lesions of donors with multiple sclerosis and in aged oligodendrocyte-lineage cells. Dor ablation in mice of both sexes results in defective myelinogenesis and remyelination. Genomic occupancy in oligodendrocytes and transcriptome profiling of the optic nerves of wild-type and Dor conditional knockout mice reveal that DOR and SOX10 co-occupy enhancers of critical myelinogenesis-associated genes including Prr18, encoding an oligodendrocyte-enriched, proline-rich factor. We show that DOR targets regulatory elements of genes responsible for α-ketoglutarate biosynthesis in mature oligodendrocytes and is essential for α-ketoglutarate production and lipid biosynthesis. Supplementation with α-ketoglutarate restores oligodendrocyte-maturation defects in Dor-deficient adult mice and improves remyelination after lysolecithin-induced demyelination and cognitive function in 17-month-old wild-type mice. Our data suggest that activation of α-ketoglutarate metabolism in mature oligodendrocytes can promote myelin production during demyelination and aging. The mechanisms underlying the ability to remyelinate in aging and disease are unclear. Here, the authors show that DOR-mediated activation of α-ketoglutarate in mature oligodendrocytes can promote myelin production in mice during demyelination and aging.
激活成熟少突胶质细胞中的 DOR 可调节α-酮戊二酸代谢,从而增强老龄小鼠的髓鞘再形成能力
成熟少突胶质细胞产生髓鞘的能力下降会对脱髓鞘疾病和衰老中的再髓鞘化产生负面影响,但其潜在机制尚不完全清楚。在本研究中,我们发现了一个成熟少突胶质细胞富集的转录核心调节因子糖尿病和肥胖相关基因(DOR)/肿瘤蛋白 p53 诱导核蛋白 2(TP53INP2),它在多发性硬化症供体的脱髓鞘病变和衰老的少突胶质细胞系细胞中下调。小鼠(雌雄均可)的髓鞘消融导致髓鞘生成和再髓鞘化缺陷。野生型小鼠和 Dor 条件性基因敲除小鼠视神经中的少突胶质细胞基因组占位和转录组图谱分析表明,DOR 和 SOX10 共同占据了关键的髓鞘生成相关基因的增强子,包括 Prr18(编码一种富含脯氨酸的少突胶质细胞因子)。我们的研究表明,DOR靶向成熟少突胶质细胞中负责α-酮戊二酸生物合成的基因的调控元件,并且对α-酮戊二酸的产生和脂质的生物合成至关重要。补充α-酮戊二酸可恢复Dor缺陷成年小鼠少突胶质细胞成熟缺陷,并改善溶血卵磷脂诱导脱髓鞘后的再髓鞘化和17个月大野生型小鼠的认知功能。我们的数据表明,激活成熟少突胶质细胞中的α-酮戊二酸代谢可促进脱髓鞘和衰老过程中的髓鞘生成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


