Energy partitioning in the cell cortex
IF 17.6
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
Abstract
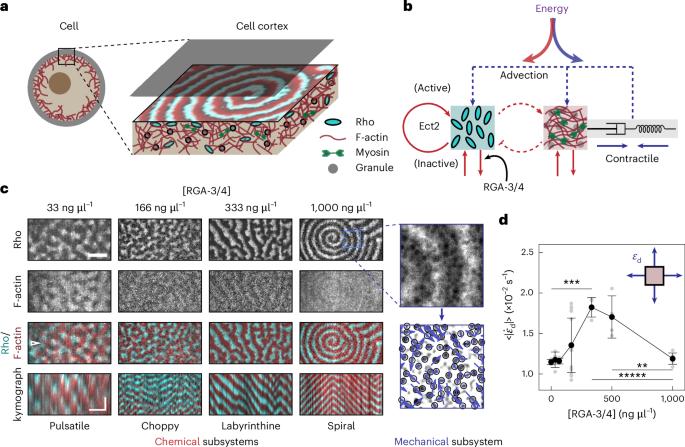
Living systems are driven far from thermodynamic equilibrium through the continuous consumption of ambient energy. In the cell cortex, this energy is invested in the formation of diverse patterns in chemical and mechanical activities, whose spatial and temporal dynamics determine the cell phenotypes and behaviours. How cells partition internal energy between these activities is unknown. Here we measured the entropy production rate of both chemical and mechanical subsystems of the cell cortex across a variety of patterns as the system is driven further from equilibrium. We do this by manipulating the Rho GTPase pathway, which controls the cortical actin filaments and myosin-II. At lower levels of GTPase-activating protein expression, which produce pulses or choppy Rho and actin filament waves, energy is proportionally partitioned between the two subsystems and is subject to the constraint of Onsager reciprocity. Within the range of reciprocity, the entropy production rate is maximized in choppy waves. As the cortex is driven into labyrinthine or spiral travelling waves, reciprocity is broken, marking an increasingly differential partitioning of energy and an uncoupling of chemical and mechanical activities. We further demonstrate that energy partitioning and reciprocity are determined by the competing timescales between chemical reaction and mechanical relaxation. How cells manage the internal energetic budget to drive mechanical and chemical dynamics is still an open question. Now it is shown that the allocation of energy depends on the distance from thermodynamic equilibrium.
细胞皮层的能量分配
生命系统通过不断消耗环境能量而远离热力学平衡。在细胞皮层中,这些能量用于形成化学和机械活动的各种模式,其空间和时间动态决定了细胞的表型和行为。细胞如何在这些活动之间分配内部能量尚不清楚。在这里,我们测量了细胞皮层的化学和机械子系统在各种模式下的熵产生率,因为系统被驱动进一步偏离平衡。我们通过操纵控制皮层肌动蛋白丝和肌球蛋白-II的 Rho GTPase 通路来实现这一目的。在较低的 GTPase 激活蛋白表达水平下,会产生脉冲或汹涌的 Rho 和肌动蛋白丝波浪,能量会在两个子系统之间按比例分配,并受到昂萨格互惠约束。在互易范围内,汹涌波的熵产生率最大。当大脑皮层被驱动进入迷宫式或螺旋式行波时,互易性被打破,标志着能量分配的差异越来越大,化学和机械活动脱钩。我们进一步证明,能量分配和互易性是由化学反应和机械松弛之间相互竞争的时间尺度决定的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


