Structural reconstruction of mouse acute aortic dissection by intravenously administered human Muse cells without immunosuppression
IF 5.4
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
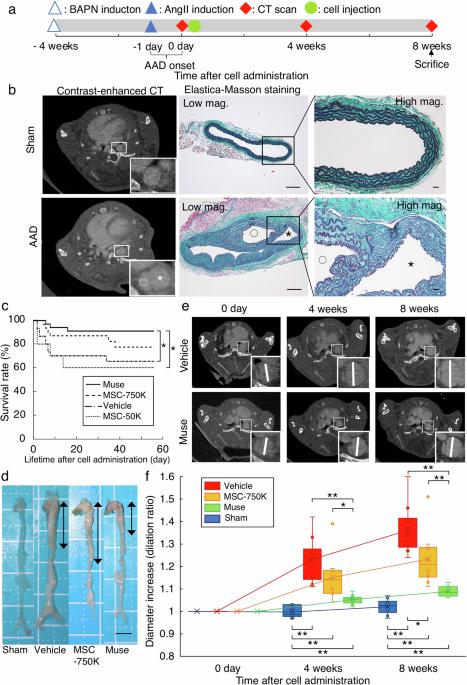
Stanford type B-acute aortic dissection (type B-AAD) is often life-threatening without invasive surgery. Multilineage-differentiating stress enduring cell (Muse cells), which comprise several percent of mesenchymal stem cells (MSCs), are endogenous pluripotent-like stem cells that selectively home to damaged tissue and replace damaged/apoptotic cells by in-vivo differentiation. Mortality, aortic diameter expansion, cell localization, cell differentiation, and inflammation of the dissected aorta were evaluated in type B-AAD model mice intravenously injected with human-Muse cells, -elastin-knockdown (KD)-Muse cells, -human leukocyte antigen-G (HLA-G)-KD-Muse cells, or MSCs, all without immunosuppressant. Here, we show the Muse (50,000 cells) group has a lower incidence of aortic rupture and mortality of AAD compared with the MSC-50K (50,000 human-MSCs) and vehicle groups. Spectrum computed tomography in-vivo dynamics and 3-dimensional histologic analyses demonstrate that Muse cells more effectively home to the AAD tissue and survive for 8 weeks in the Muse group than in the MSC-750K (750,000 human-MSCs containing 50,000 Muse cells) group. Homing of Muse cells is impeded in the HLA-G-KD-Muse (50,000 cells) group. Differentiation of homed Muse cells into CD31(+) and alpha-smooth muscle actin (+) cells, production and reorganization of elastic fibers in the AAD tissue, and suppression of diameter expansion are greater in the Muse group than in the MSC-750K and elastin-KD-Muse (50,000 cells) groups. Intravenously administered Muse cells reconstruct the dissected aorta and improve mortality and diameter enlargement rates. Moreover, small doses of purified Muse cells are more effective than large doses of MSCs. HLA-G is suggested to contribute to the successful survival and homing of Muse cells. Acute aortic dissection (AAD) is a serious disease in which the largest artery in the body, called the aorta, enlarges and ruptures. Surgery is often required to prevent death. Cells called Muse cells have been injected into people during clinical trials to treat other diseases. In this study, we injected Muse cells into mice with dissected aorta. The cells accumulated in damaged parts of the aorta and strengthened the structure of the aorta, reducing the number of mice that died. If further research shows this treatment works in humans, this could enable AAD to be treated without surgery and potentially improve the treatment and survival of people with AAD. Takahashi et al. intravenously inject human Muse cells into model mice with Stanford type B acute aortic dissection. This effectively reduces diameter expansion rates, mortality and has a greater therapeutic effect than intravenous injection of large amounts of human mesenchymal stem cells.
通过静脉注射人类 Muse 细胞重建小鼠急性主动脉夹层的结构而无需免疫抑制
斯坦福B型急性主动脉夹层(B型-AAD)如果不进行侵入性手术,往往会危及生命。多系分化应激持久细胞(Muse细胞)占间充质干细胞(MSCs)的百分之几,是一种内源性多能样干细胞,可选择性地进入受损组织,并通过体内分化取代受损/凋亡细胞。在B-AAD模型小鼠体内静脉注射人-Muse细胞、elastin-敲除(KD)-Muse细胞、人白细胞抗原-G(HLA-G)-KD-Muse细胞或间叶干细胞后,评估了死亡率、主动脉直径扩张、细胞定位、细胞分化和断裂主动脉的炎症情况,所有实验均未使用免疫抑制剂。在这里,我们发现与 MSC-50K(50,000 人-间充质干细胞)组和载体组相比,Muse(50,000 细胞)组的主动脉破裂发生率和 AAD 死亡率较低。频谱计算机断层扫描体内动力学和三维组织学分析表明,与 MSC-750K(75 万人类间充质干细胞,含 5 万 Muse 细胞)组相比,Muse 组的 Muse 细胞能更有效地归巢到 AAD 组织,并存活 8 周。HLA-G-KD-Muse(50,000 个细胞)组的 Muse 细胞归巢受阻。与 MSC-750K 组和弹性蛋白-KD-Muse(50,000 个细胞)组相比,Muse 组的归巢 Muse 细胞分化为 CD31(+)细胞和α-平滑肌肌动蛋白(+)细胞,AAD 组织中弹性纤维的生成和重组以及直径扩张的抑制作用更强。静脉注射 Muse 细胞可重建断裂的主动脉,提高死亡率和直径扩大率。此外,小剂量的纯化 Muse 细胞比大剂量的间充质干细胞更有效。HLA-G被认为有助于Muse细胞的成功存活和归巢。急性主动脉夹层(AAD)是一种严重疾病,人体内最大的动脉(主动脉)在这种情况下会发生扩张和破裂。通常需要通过手术来防止死亡。在治疗其他疾病的临床试验中,人们已经注射了名为Muse细胞的细胞。在这项研究中,我们将缪斯细胞注射到主动脉断裂的小鼠体内。细胞在主动脉受损部位聚集,强化了主动脉的结构,减少了小鼠的死亡数量。如果进一步的研究表明这种治疗方法对人类有效,那么就可以在不进行手术的情况下治疗主动脉瓣狭窄,并有可能改善主动脉瓣狭窄患者的治疗和存活率。高桥等人将人类缪斯细胞静脉注射到患有斯坦福B型急性主动脉夹层的模型小鼠体内。与静脉注射大量人类间充质干细胞相比,这能有效降低直径扩张率和死亡率,并具有更大的治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


