High-risks drug adverse events associated with Cetirizine and Loratadine for the treatment of allergic diseases: A retrospective pharmacovigilance study based on the FDA adverse event reporting system database
Abstract
Background
Cetirizine and Loratadine are the two best-selling second-generation antihistamines for allergic diseases. This study aims to provide a comparative analysis of the differences in adverse drug events (ADEs) between these two medications, which can assist clinicians in making appropriate treatment decisions.
Methods
ADE reports related to Cetirizine and Loratadine obtained from the FDA adverse event reporting system (FAERS) database were analyzed using disproportionality analysis and Bayesian analysis to evaluate and compare the ADE signals of both drugs.
Results
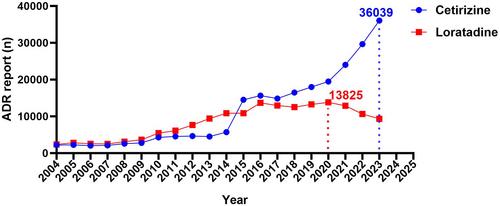
A total of 28,051 and 28,073 ADE reports were retrieved from the FAERS database related to Cetirizine and Loratadine, respectively, with both drugs showing a predominance of middle-aged females. Specifically, Loratadine was associated with respiratory symptoms, mainly nasal symptoms such as rhinorrhea (n = 326, ROR 6.75), sneezing (n = 251, ROR 15.24), and nasal congestion (n = 185, ROR 4.25), while Cetirizine did not show this association. Notably, both drugs exhibited strong signals for somnolence in the nervous and psychiatric systems, especially Cetirizine (Cetirizine, n = 2556, ROR 10.52 vs. Loratadine, n = 1200, ROR 7.76). Additionally, Cetirizine itself showed strong signals for attention disturbance (n = 233, ROR 3.3), while Loratadine was associated with nervousness (n = 145, ROR 3.3). Further exploration revealed more severe adverse reactions closely associated with Cetirizine, including hallucinations, aggression, and abnormal behavior. Importantly, Cetirizine was significantly associated with the occurrence of pericarditis (n = 138, ROR 8.13), potentially leading to serious adverse consequences.
Conclusion
Compared to Loratadine, Cetirizine poses a greater potential risk in the nervous and psychiatric systems. Additionally, this study reveals previously underestimated potential cardiac toxicity of Cetirizine; albeit at a relatively low incidence rate, the high signal intensity warrants further attention and exploration. These findings highlight the need for enhanced patient monitoring and therapy optimization when prescribing these medications, ensuring better management of allergic diseases while minimizing risks.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


