On the Structure Sensitivity of CO2 Hydrogenation over Cu/ZrO2: Insights into the Role of the Support and the Active Sites
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
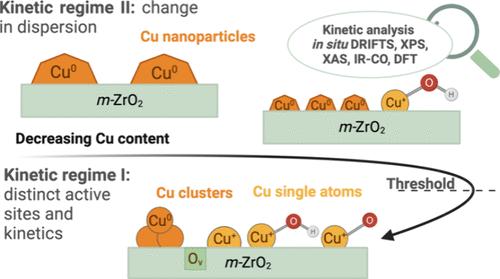
The well-known structure sensitivity of CO2 hydrogenation to methanol has shown to be an impactful topic for the performance of the catalyst and yet remains unaddressed for Cu nanoparticles supported on ZrO2, a material that has shown to be involved in the active site and the reaction mechanism of methanol formation. Herein, Cu/ZrO2 catalysts were studied to unravel the underlying structure–activity relationships by combining surface and bulk characterization techniques, kinetic measurements, operando-DRIFTS and DFT calculations. Contrary to Cu over inert supports, the results showed different trends and two distinct kinetic regimes. For Cu nanoparticles larger than 2 nm, they are in accordance with previously reported results, this is, a change in the number of active sites, without affecting the nature of them. Conversely, it is demonstrated that the active sites are markedly different over the regime of nanoparticles smaller than 2 nm, accessed for ultralow Cu contents of 0.1 wt %, as evidenced from the systematic change of kinetic parameters and from operando-DRIFTS. The distinct active sites were identified as isolated Cu species (i.e., single atoms and Cu incorporated into the lattice of ZrO2) and highly stable Cu clusters, both of which would allocate the formation of products for low metal contents. The results are certainly related to the interaction between Cu and ZrO2 and unequivocally disclose the relationship between activity regimes and the nature of active sites as a function of the Cu particle size. Furthermore, they demonstrate that distinct active sites can be accessed just by varying the metal content on active reducible supports. As such, the findings are of particular relevance for the fundamental understanding of the interaction between Cu and ZrO2 and its interdependence with the size of the Cu nanoparticles, as well as for the rational design of catalysts for CO2 hydrogenation to methanol.
关于 Cu/ZrO2 上 CO2 加氢反应的结构敏感性:透视支撑和活性位点的作用
众所周知,二氧化碳加氢制甲醇的结构敏感性是影响催化剂性能的一个重要问题,但对于 ZrO2 上支撑的铜纳米颗粒来说,这一问题仍未得到解决,而这种材料已被证明参与了甲醇形成的活性位点和反应机理。在此,我们对 Cu/ZrO2 催化剂进行了研究,通过结合表面和块体表征技术、动力学测量、Operando-DRIFTS 和 DFT 计算来揭示其潜在的结构-活性关系。与惰性载体上的铜催化剂相反,研究结果显示出不同的趋势和两种截然不同的动力学机制。对于大于 2 纳米的铜纳米粒子,其结果与之前报道的结果一致,即活性位点的数量发生了变化,但并不影响其性质。相反,从动力学参数的系统变化和操作-DRIFTS 可以看出,活性位点在小于 2 纳米的纳米颗粒体系中明显不同,在铜含量为 0.1 wt % 的超低纳米颗粒中也是如此。不同的活性位点被确定为孤立的铜物种(即单原子和铜结合到 ZrO2 的晶格中)和高度稳定的铜簇,这两种位点都能形成低金属含量的产物。这些结果肯定与铜和 ZrO2 之间的相互作用有关,并明确揭示了活性机制与活性位点性质之间的关系,即铜粒度的函数关系。此外,这些研究还表明,只需改变活性还原支撑物上的金属含量,就能获得不同的活性位点。因此,这些发现对于从根本上理解铜和 ZrO2 之间的相互作用及其与铜纳米粒子大小的相互依存关系,以及合理设计二氧化碳加氢制甲醇催化剂具有特别重要的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


