Gasdermin D-mediated metabolic crosstalk promotes tissue repair
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
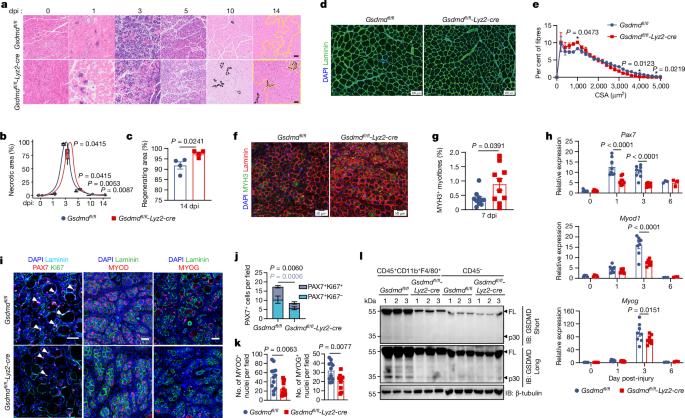
The establishment of an early pro-regenerative niche is crucial for tissue regeneration1,2. Gasdermin D (GSDMD)-dependent pyroptosis accounts for the release of inflammatory cytokines upon various insults3–5. However, little is known about its role in tissue regeneration followed by homeostatic maintenance. Here we show that macrophage GSDMD deficiency delays tissue recovery but has little effect on the local inflammatory milieu or the lytic pyroptosis process. Profiling of the metabolite secretome of hyperactivated macrophages revealed a non-canonical metabolite-secreting function of GSDMD. We further identified 11,12-epoxyeicosatrienoic acid (11,12-EET) as a bioactive, pro-healing oxylipin that is secreted from hyperactive macrophages in a GSDMD-dependent manner. Accumulation of 11,12-EET by direct supplementation or deletion of Ephx2, which encodes a 11,12-EET-hydrolytic enzyme, accelerated muscle regeneration. We further demonstrated that EPHX2 accumulated within aged muscle, and that consecutive 11,12-EET treatment rejuvenated aged muscle. Mechanistically, 11,12-EET amplifies fibroblast growth factor signalling by modulating liquid–liquid phase separation of fibroblast growth factors, thereby boosting the activation and proliferation of muscle stem cells. These data depict a GSDMD-guided metabolite crosstalk between macrophages and muscle stem cells that governs the repair process, which offers insights with therapeutic implications for the regeneration of injured or aged tissues. Untargeted metabolomics reveals that 11,12-epoxyeicosatrienoic acid released from macrophages via gasdermin G pores has a key role in promoting fibroblast growth factor-dependent muscle regeneration following injury.
Gasdermin D 介导的新陈代谢串联促进组织修复
早期促再生龛位的建立对组织再生至关重要1,2。依赖 Gasdermin D(GSDMD)的热裂解是各种损伤时释放炎性细胞因子的原因3-5。然而,人们对其在组织再生和平衡维持中的作用知之甚少。在这里,我们发现巨噬细胞 GSDMD 缺乏会延迟组织的恢复,而对局部炎症环境或裂解性热蛋白沉积过程几乎没有影响。超活化巨噬细胞的代谢物分泌组图谱揭示了 GSDMD 的非经典代谢物分泌功能。我们进一步确定了 11,12-epoxyeicosatrienoic acid(11,12-EET)是一种具有生物活性的促进愈合的氧脂素,它以一种依赖于 GSDMD 的方式从高活性巨噬细胞中分泌。事实上,通过直接补充或删除其水解酶 Ephx2 来积累 11,12-EET 可加速肌肉再生。我们进一步证实,Ephx2水平在老化肌肉中累积。连续的 11,12-EET 治疗可使老化肌肉恢复活力。从机理上讲,11,12-EET 通过调节成纤维细胞生长因子的液相-液相分离,扩大了成纤维细胞生长因子-成纤维细胞生长因子受体的信号转导,从而促进了肌肉干细胞(MuSCs)的活化和增殖。这些数据描述了巨噬细胞和肌肉干细胞之间由 GSDMD 引导的代谢物串扰,这种串扰控制着修复过程,为损伤或老化组织的再生提供了新的治疗思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


