Study on Continuous-Flow Process for Direct Synthesis of p-Aminophenol from Nitrobenzene
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
p-Aminophenol (PAP) is an important organic chemical raw material and a pharmaceutical intermediate. Catalytic hydrogenation of nitrobenzene (NB) is an environmentally friendly and economical production method. However, the one-pot method in a traditional batch reactor often leads to a low reaction rate and low PAP yield at low hydrogen pressure. In this work, a continuous-flow process for direct synthesis of PAP by the hydrogenation–rearrangement of NB was established, which provides a safe, green, and efficient method for the synthesis of PAP. The effects of various reaction conditions were investigated. Under the optimal reaction conditions, a 94.5% yield of phenylhydroxylamine (PHA) was achieved in the hydrogenation process under atmospheric pressure. The catalyst activity remained good for 50 h of continuous operation. Solvent tetrahydrofuran (THF) and additive 4-dimethylaminopyridine (DMAP) are more conducive to the synthesis of PHA than other solvents. For different acid catalysts in the Bamberger rearrangement with an equivalent concentration of 2 N, stronger acidity led to greater conversion of PHA. The Bamberger rearrangement is solvent-sensitive, and aprotic solvents will reduce the conversion of PHA. The full continuous process for direct synthesis of PAP from NB was studied by mixing sulfuric acid solution and PHA/THF solution with a microfluidic chip. The conversion of PHA was 100% with a low H2SO4 concentration of 1 wt % at a residence time of 13.6 min. The process was reduced from the hour level of the batch process to the minute level, and the H2SO4 concentration was reduced.
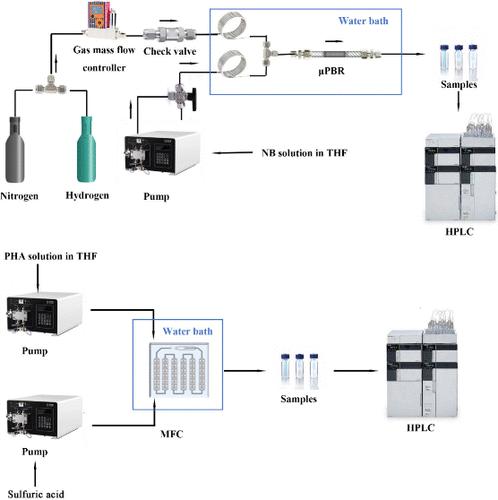
从硝基苯直接合成对氨基苯酚的连续流工艺研究
对氨基苯酚(PAP)是一种重要的有机化工原料和医药中间体。硝基苯(NB)催化加氢是一种环保、经济的生产方法。然而,在传统的间歇反应器中采用单锅法往往会导致反应速率较低,在低氢压条件下 PAP 收率较低。本研究建立了一种通过 NB 加氢重排直接合成 PAP 的连续流工艺,为 PAP 的合成提供了一种安全、绿色和高效的方法。研究了各种反应条件的影响。在最佳反应条件下,常压下氢化过程的苯羟胺(PHA)产率达到 94.5%。催化剂在连续运行 50 小时后仍保持良好的活性。与其他溶剂相比,溶剂四氢呋喃(THF)和添加剂 4-二甲氨基吡啶(DMAP)更有利于 PHA 的合成。对于班伯格重排中的不同酸催化剂(2 N 浓度相等),酸性越强,PHA 的转化率越高。班伯格重排对溶剂敏感,钝性溶剂会降低 PHA 的转化率。通过在微流控芯片中混合硫酸溶液和 PHA/THF 溶液,研究了从 NB 直接合成 PAP 的全连续过程。在 H2SO4 浓度为 1 wt % 的低浓度条件下,PHA 的转化率为 100%,停留时间为 13.6 分钟。该过程从间歇过程的小时级降至分钟级,H2SO4 浓度也降低了。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


