Substrate interactions guide cyclase engineering and lasso peptide diversification
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
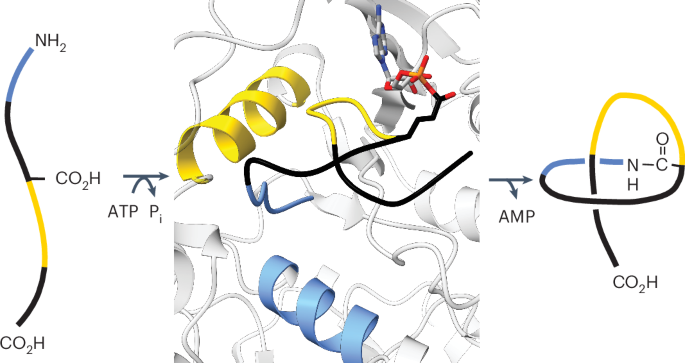
Lasso peptides are a diverse class of naturally occurring, highly stable molecules kinetically trapped in a distinctive [1]rotaxane conformation. How the ATP-dependent lasso cyclase constrains a relatively unstructured substrate peptide into a low entropy product has remained a mystery owing to poor enzyme stability and activity in vitro. In this study, we combined substrate tolerance data with structural predictions, bioinformatic analysis, molecular dynamics simulations and mutational scanning to construct a model for the three-dimensional orientation of the substrate peptide in the lasso cyclase active site. Predicted peptide cyclase molecular contacts were validated by rationally engineering multiple, phylogenetically diverse lasso cyclases to accept substrates rejected by the wild-type enzymes. Finally, we demonstrate the utility of lasso cyclase engineering by robustly producing previously inaccessible variants that tightly bind to integrin αvβ8, which is a primary activator of transforming growth factor β and, thus, an important anti-cancer target. How a lasso cyclase ties a lasso peptide into its characteristic knot has remained poorly understood. Here the authors identify key molecular interactions that guide lasso peptide folding and cyclase substrate tolerance to inform cyclase engineering for expanded lasso peptide diversity.

底物相互作用引导环化酶工程和套索肽多样化
拉索肽是一类天然存在的多种多样的高稳定性分子,它们在动力学上被困在一种独特的 [1]rotaxane 构象中。由于体外酶的稳定性和活性较差,依赖 ATP 的拉索环化酶如何将相对非结构化的底物肽限制为低熵产物一直是个谜。在这项研究中,我们将底物耐受性数据与结构预测、生物信息分析、分子动力学模拟和突变扫描相结合,构建了底物肽在拉索环化酶活性位点中的三维取向模型。通过对多种系统发育不同的拉索环化酶进行合理工程设计,使其接受被野生型酶拒绝的底物,从而验证了预测的肽环化酶分子接触。最后,我们证明了拉索环化酶工程学的实用性,它能产生以前无法获得的变体,与整合素αvβ8紧密结合,而整合素αvβ8是转化生长因子β的主要激活剂,因此是重要的抗癌靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


