A randomized, double-blind, placebo-controlled, dose-escalating phase IIa trial to evaluate the safety, tolerability, efficacy, and pharmacokinetics of multiple oral doses of Pynegabine tablets as add-on therapy in patients with focal epilepsy
Abstract
Aims
This study aims to investigate the safety, tolerability, efficacy, and pharmacokinetics of Pynegabine as an add-on therapy in the treatment of focal epilepsy.
Methodology
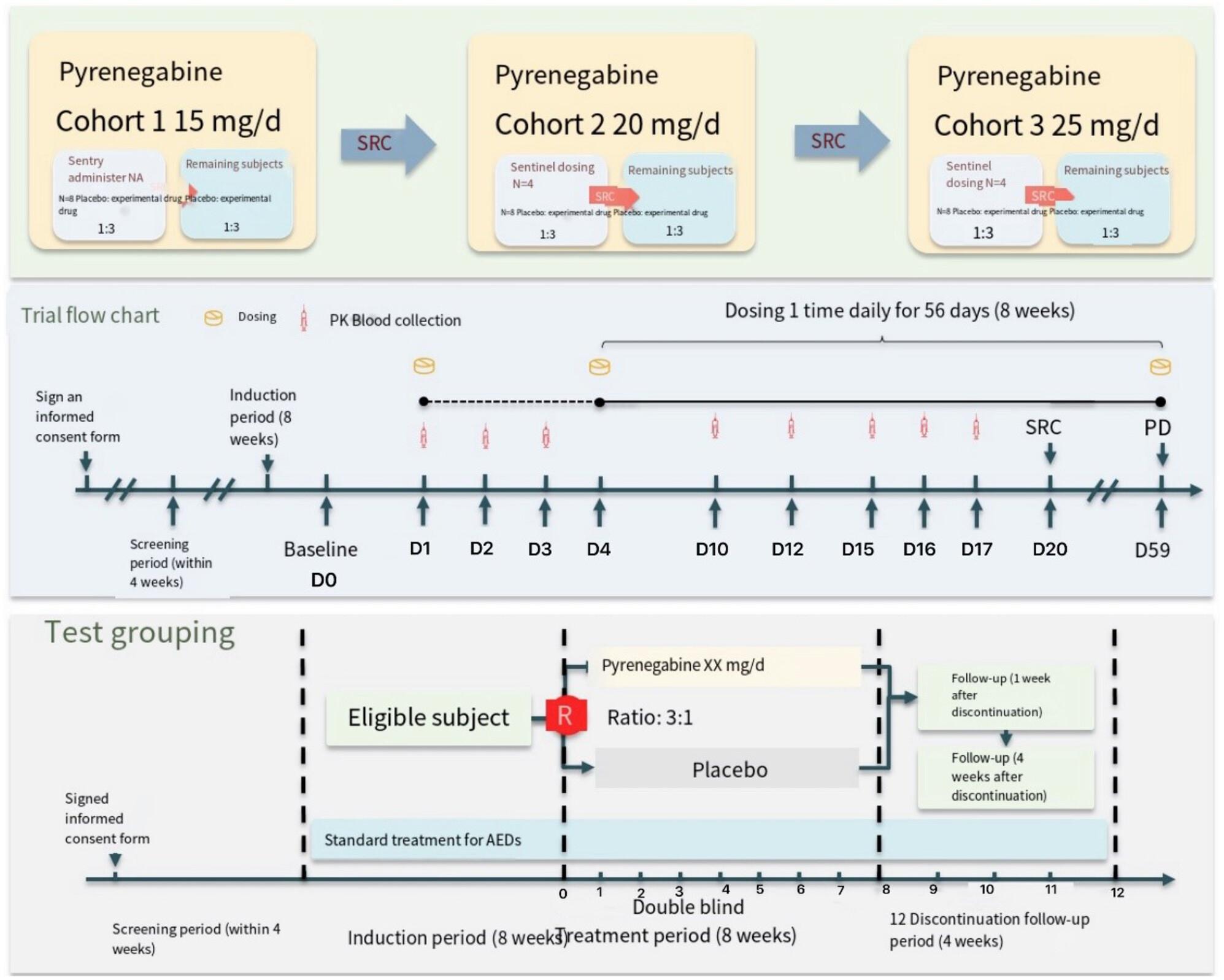
This is a protocol phase-IIa, randomized, double-blinded, placebo-controlled, multicenter study in patients with focal epilepsy from multiple centers in China who have been treated with at least 2 ASMs without effective control. The study involves an 8-week run-in period with stable use of previous medications. Patients are then randomized to receive either Pynegabine or a placebo. Sentinel administration is performed initially, and subsequent patients are randomized based on safety assessments. Three dose cohorts (15, 20, and 25 mg/d) are established. Efficacy is assessed through various measures, including seizure frequency, CGI score, PGI score, HAMA score, HAMD score, MoCA scale score, QOLIE-31 scale score, and 12 h-EEG score. Safety evaluations, PK blood samples, concomitant medications, and adverse events are also recorded.
Conclusion
Data from the study will be used to evaluate the safety, tolerability, efficacy, and pharmacokinetics of Pynegabine tablets as add-on therapy for focal epilepsy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


