Digitonin-Loaded Nanoscale Metal–Organic Framework for Mitochondria-Targeted Radiotherapy-Radiodynamic Therapy and Disulfidptosis
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The efficacy of radiotherapy (RT) is limited by inefficient X-ray absorption and reactive oxygen species generation, upregulation of immunosuppressive factors, and a reducing tumor microenvironment (TME). Here, the design of a mitochondria-targeted and digitonin (Dig)-loaded nanoscale metal-organic framework, Th-Ir-DBB/Dig, is reported to overcome these limitations and elicit strong antitumor effects upon low-dose X-ray irradiation. Built from Th6O4(OH)4 secondary building units (SBUs) and photosensitizing Ir(DBB)(ppy)22+ (Ir-DBB, DBB = 4,4′-di(4-benzoato)−2,2′-bipyridine; ppy = 2-phenylpyridine) ligands, Th-Ir-DBB exhibits strong RT-radiodynamic therapy (RDT) effects via potent radiosensitization with high-Z SBUs for hydroxyl radical generation and efficient excitation of Ir-DBB ligands for singlet oxygen production. Th-Ir-DBB/Dig releases digitonin in acidic TMEs to trigger disulfidptosis of cancer cells and sensitize cancer cells to RT-RDT through glucose and glutathione depletion. The released digitonin simultaneously downregulates multiple immune checkpoints in cancer cells and T cells through cholesterol depletion. As a result, Th-Ir-DBB/dig plus X-ray irradiation induces strong antitumor immunity to effectively inhibit tumor growth in mouse models of colon and breast cancer.
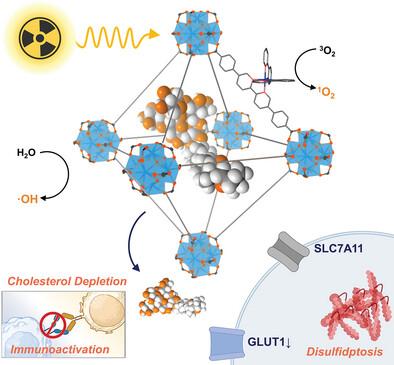
用于线粒体靶向放疗--放射动力疗法和二硫化钼的地高辛负载纳米级金属有机框架
放疗(RT)的疗效受限于对 X 射线的低效吸收和活性氧的生成、免疫抑制因子的上调以及肿瘤微环境(TME)的降低。本文报告了一种靶向线粒体并负载地高辛(Dig)的纳米级金属有机框架 Th-Ir-DBB/Dig,它的设计克服了这些局限性,并在低剂量 X 射线照射下产生强烈的抗肿瘤效应。该框架由 Th6O4(OH)4 二级构建单元(SBU)和光敏性 Ir(DBB)(ppy)22+ (Ir-DBB,DBB = 4,4′-二(4-苯甲酸)-2,2′-联吡啶;ppy=2-苯基吡啶)配体,Th-Ir-DBB 通过高 Z SBU 产生羟基自由基和 Ir-DBB 配体产生单线态氧的高效激发,表现出强大的 RT 放射治疗(RDT)效果。Th-Ir-DBB/Dig能在酸性TME中释放地高宁,引发癌细胞脱硫,并通过葡萄糖和谷胱甘肽耗竭使癌细胞对RT-RDT敏感。释放的地高辛同时通过胆固醇消耗,下调癌细胞和 T 细胞中的多个免疫检查点。因此,Th-Ir-DBB/dig 加上 X 射线照射可诱导强大的抗肿瘤免疫力,从而有效抑制结肠癌和乳腺癌小鼠模型中肿瘤的生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


