Engineered Nanoparticles for Enhanced Antitumoral Synergy Between Macrophages and T Cells in the Tumor Microenvironment
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
T cells and macrophages have the potential to collaborate to eliminate tumor cells efficiently. Macrophages can eliminate tumor cells through phagocytosis and subsequently activate T cells by presenting tumor antigens. The activated T cells, in turn, can kill tumor cells and redirect tumor-associated macrophages toward an antitumoral M1 phenotype. However, checkpoint molecules expressed on tumor cells impede the collaborative action of these immune cells. Meanwhile, monotherapy with a single immune checkpoint inhibitor (ICI) for either macrophages or T cells yields suboptimal efficacy in cancer patients. To address this challenge, here a nanoparticle capable of efficiently delivering dual ICIs to tumors for both macrophages and T cells is developed. These programmed cell death protein 1 (PD-1)-transfected macrophage membrane-derived nanoparticles (PMMNPs) can target tumors and provide signal-regulatory protein alpha and PD-1 to block CD47 and programmed cell death-ligand 1 (PD-L1), respectively, on tumor cells. PMMNPs enhance macrophage-mediated cancer cell phagocytosis and antigen presentation, promote T cell activation, and induce the reprogramming of macrophages toward an antitumoral phenotype. In syngeneic tumor-bearing mice, PMMNPs demonstrate superior therapeutic efficacy compared to nanoparticles delivering single ICIs and non-targeted delivery of anti-CD47 and anti-PD-L1 antibodies. PMMNPs capable of augmenting the antitumoral interplay between macrophages and T cells may offer a promising avenue for cancer immunotherapy.
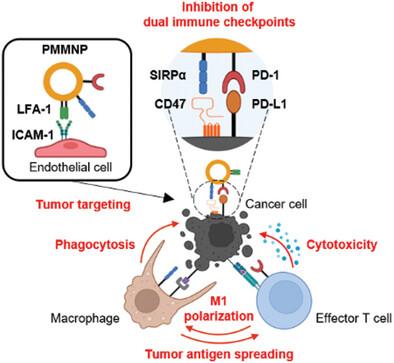
增强肿瘤微环境中巨噬细胞和 T 细胞抗肿瘤协同作用的工程纳米粒子
T 细胞和巨噬细胞有可能合作有效地消灭肿瘤细胞。巨噬细胞可以通过吞噬作用消灭肿瘤细胞,然后通过呈现肿瘤抗原激活 T 细胞。活化的 T 细胞反过来又能杀死肿瘤细胞,并将肿瘤相关巨噬细胞重新导向抗肿瘤的 M1 表型。然而,肿瘤细胞上表达的检查点分子会阻碍这些免疫细胞的协同作用。同时,使用单一免疫检查点抑制剂(ICI)对巨噬细胞或T细胞进行单药治疗,对癌症患者的疗效并不理想。为了应对这一挑战,我们在此开发了一种纳米粒子,它能够向肿瘤中的巨噬细胞和T细胞高效递送双重ICI。这些经程序性细胞死亡蛋白1(PD-1)转染的巨噬细胞膜衍生纳米粒子(PMMNPs)可以靶向肿瘤,提供信号调节蛋白α和PD-1,分别阻断肿瘤细胞上的CD47和程序性细胞死亡配体1(PD-L1)。PMMNPs 可增强巨噬细胞介导的癌细胞吞噬能力和抗原呈递能力,促进 T 细胞活化,并诱导巨噬细胞向抗肿瘤表型重编程。与递送单一 ICIs 的纳米颗粒以及非靶向递送抗 CD47 和抗 PD-L1 抗体相比,PMMNPs 在注射肿瘤小鼠中显示出更优越的疗效。能够增强巨噬细胞和T细胞之间抗肿瘤相互作用的PMMNPs可能会为癌症免疫疗法提供一种前景广阔的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


