Unravelling the Impact of Process Impurities on the Crystallization of Ritlecitinib Tosylate Using Molecular Dynamics
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
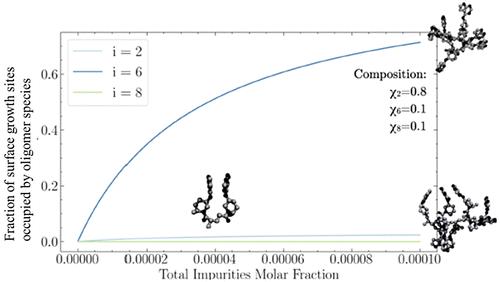
We investigate the influence of oligomeric impurities on the crystallization of ritlecitinib tosylate, an active pharmaceutical compound, using a combined experimental and molecular modeling approach. Ritlecitinib oligomers, particularly hexamers, were identified as key species hindering crystal growth. Experimental outcomes highlighted the inhibitory effects of oligomers on crystallization kinetics, yield, and physical properties. Simplified free energy methods based on the linear interaction energy model revealed a nonmonotonic relationship between oligomer size and surface affinity, with hexamers having the most prominent tendency to block the surface of ritlecitinib tosylate crystals, thus impacting crystal growth. A competitive Langmuir adsorption isotherm model quantified the reduction in crystal growth rates due to oligomer adsorption, providing a systematic approach to understanding these inhibitory effects. This research enhances our understanding of the molecular mechanisms governing oligomer adsorption, and more generally, impurity adsorption, on crystal surfaces and offers insights for designing crystal growth inhibitors in pharmaceutical applications.
利用分子动力学揭示工艺杂质对瑞替西替尼对甲苯磺酸盐结晶的影响
我们采用实验和分子建模相结合的方法,研究了低聚物杂质对活性药物化合物瑞替西替尼对甲苯磺酸盐结晶的影响。研究发现,瑞替西替尼低聚物,尤其是六聚物,是阻碍晶体生长的关键物种。实验结果凸显了低聚物对结晶动力学、产量和物理性质的抑制作用。基于线性相互作用能模型的简化自由能方法揭示了低聚物大小与表面亲和力之间的非单调关系,其中六聚物最有可能阻塞托西酸瑞替西替尼晶体的表面,从而影响晶体生长。竞争性朗缪尔吸附等温线模型量化了低聚物吸附导致的晶体生长速率降低,为理解这些抑制作用提供了一种系统方法。这项研究加深了我们对晶体表面低聚物吸附以及更广泛的杂质吸附的分子机制的理解,并为设计制药应用中的晶体生长抑制剂提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


