Distinct mucosal endotypes as initiators and drivers of rheumatoid arthritis
IF 32.7
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
Abstract
Rheumatoid arthritis (RA) is a potentially devastating autoimmune disease. The great majority of patients with RA are seropositive for anti-citrullinated protein antibodies (ACPAs), rheumatoid factors, or other autoantibodies. The onset of clinically apparent inflammatory arthritis meeting classification criteria (clinical RA) is preceded by ACPA seropositivity for an average of 3–5 years, a period that is designated as ‘at-risk’ of RA for ACPA-positive individuals who do not display signs of arthritis, or ‘pre-RA’ for individuals who are known to have progressed to developing clinical RA. Prior studies of individuals at-risk of RA have associated pulmonary mucosal inflammation with local production of ACPAs and rheumatoid factors, leading to development of the ‘mucosal origins hypothesis’. Recent work now suggests the presence of multiple distinct mucosal site-specific mechanisms that drive RA evolution. Indicatively, subsets of individuals at-risk of RA and patients with RA harbour a faecal bacterial strain that has exhibited arthritogenic activity in animal models and that favours T helper 17 (TH17) cell responses in patients. Periodontal inflammation and oral microbiota have also been suggested to promote the development of arthritis through breaches in the mucosal barrier. Herein, we argue that mucosal sites and their associated microbial strains can contribute to RA evolution via distinct pathogenic mechanisms, which can be considered causal mucosal endotypes. Future therapies instituted for prevention in the at-risk period, or, perhaps, during clinical RA as therapeutics for active arthritis, will possibly have to address these individual mechanisms as part of precision medicine approaches. Holers and colleagues review current data linking immune mechanisms and dysbiosis at distinct mucosal sites to risk of rheumatoid arthritis (RA). Their newly introduced causal mucosal endotypes hypothesis suggests that lung-, gut-, or oral-associated endotypes might drive the pathogenesis and progression of RA, and highlights associated research directions towards preventive and therapeutic strategies in RA.
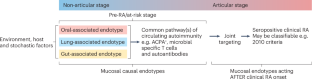
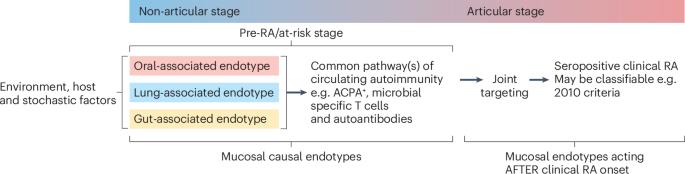
作为类风湿性关节炎诱因和驱动因素的不同粘膜内型
类风湿性关节炎(RA)是一种具有潜在破坏性的自身免疫性疾病。绝大多数 RA 患者的抗瓜氨酸蛋白抗体(ACPA)、类风湿因子或其他自身抗体血清反应呈阳性。符合分类标准(临床 RA)的临床明显炎症性关节炎发病前,ACPA 血清阳性反应平均持续 3-5 年,这段时间对于未显示关节炎症状的 ACPA 阳性个体而言,被称为 RA "高危 "期;对于已知已发展为临床 RA 的个体而言,则称为 "RA 前 "期。先前对有 RA 风险的个体进行的研究将肺粘膜炎症与 ACPA 和类风湿因子的局部产生联系起来,从而提出了 "粘膜起源假说"。最近的研究表明,存在多种不同的粘膜部位特异性机制,驱动着RA的演变。有迹象表明,RA 高危人群和 RA 患者的粪便中含有一种细菌菌株,这种菌株在动物模型中具有致关节炎活性,在患者体内有利于 T 辅助细胞 17(TH17)的反应。牙周炎症和口腔微生物群也被认为通过破坏粘膜屏障促进关节炎的发展。在此,我们认为粘膜部位及其相关的微生物菌株可通过不同的致病机制促进关节炎的演变,这些机制可被视为致病粘膜内型。作为精准医疗方法的一部分,未来用于风险期预防的疗法,或作为活动性关节炎的临床RA治疗方法,可能必须解决这些不同机制的问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


