Stepwise de novo establishment of inactive X chromosome architecture in early development
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
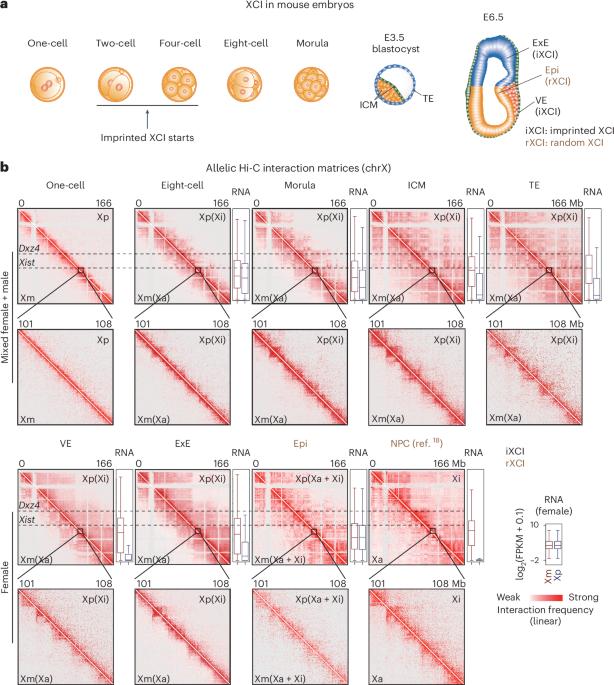
X chromosome inactivation triggers a dramatic reprogramming of transcription and chromosome architecture. However, how the chromatin organization of inactive X chromosome is established de novo in vivo remains elusive. Here, we identified an Xist-separated megadomain structure (X-megadomains) on the inactive X chromosome in mouse extraembryonic lineages and extraembryonic endoderm (XEN) cell lines, and transiently in the embryonic lineages, before Dxz4-delineated megadomain formation at later stages in a strain-specific manner. X-megadomain boundary coincides with strong enhancer activities and cohesin binding in an Xist regulatory region required for proper Xist activation in early embryos. Xist regulatory region disruption or cohesin degradation impaired X-megadomains in extraembryonic endoderm cells and caused ectopic activation of regulatory elements and genes near Xist, indicating that cohesin loading at regulatory elements promotes X-megadomains and confines local gene activities. These data reveal stepwise X chromosome folding and transcriptional regulation to achieve both essential gene activation and global silencing during the early stages of X chromosome inactivation. Hi-C analysis identifies Xist-separated megadomains (X-megadomains) on the inactive X chromosome in mouse early embryos. Cohesin loading at Xist regulatory elements promotes X-megadomain formation and restricts nearby gene activity.
在早期发育过程中逐步从头建立无活性的 X 染色体结构
X 染色体失活会引发转录和染色体结构的急剧重塑。然而,非活性 X 染色体的染色质组织是如何在体内从头建立起来的,仍然是一个未知数。在这里,我们在小鼠胚外系和胚外内胚层(XEN)细胞系中鉴定出了非活性 X 染色体上的 Xist 分离巨域结构(X-megadomains),并在胚胎系中鉴定出了瞬时结构,然后在后期以株系特异性的方式鉴定出了 Dxz4 划线的巨域形成。X-巨域边界与早期胚胎中适当激活 Xist 所需的 Xist 调节区中的强增强子活动和粘合素结合相吻合。Xist调控区的破坏或内聚素降解损害了胚外内胚层细胞中的X-megadomains,并导致调控元件和Xist附近基因的异位激活,这表明内聚素在调控元件上的负载促进了X-megadomains并限制了局部基因的活动。这些数据揭示了在X染色体失活的早期阶段,X染色体逐步折叠和转录调控实现了重要基因激活和全局沉默。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


