VEGF-A-mediated venous endothelial cell proliferation results in neoangiogenesis during neuroinflammation
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
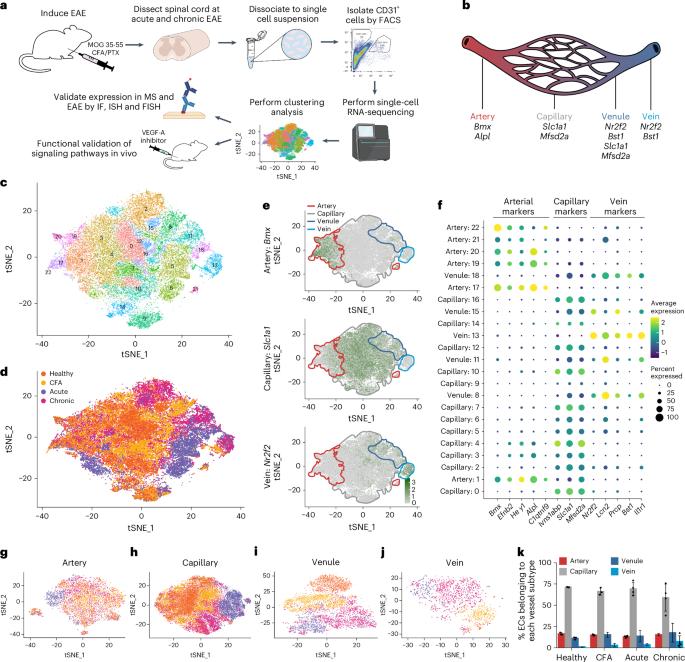
Newly formed leaky vessels and blood–brain barrier (BBB) damage are present in demyelinating acute and chronic lesions in multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE). However, the endothelial cell subtypes and signaling pathways contributing to these leaky neovessels are unclear. Here, using single-cell transcriptional profiling and in vivo validation studies, we show that venous endothelial cells express neoangiogenesis gene signatures and show increased proliferation resulting in enlarged veins and higher venous coverage in acute and chronic EAE lesions in female adult mice. These changes correlate with the upregulation of vascular endothelial growth factor A (VEGF-A) signaling. We also confirmed increased expression of neoangiogenic markers in acute and chronic human MS lesions. Treatment with a VEGF-A blocking antibody diminishes the neoangiogenic transcriptomic signatures and vascular proliferation in female adult mice with EAE, but it does not restore BBB function or ameliorate EAE pathology. Our data demonstrate that venous endothelial cells contribute to neoangiogenesis in demyelinating neuroinflammatory conditions. Defective neoangiogenesis and blood–brain barrier leakiness are pathological hallmarks of neuroinflammation. Here the authors show that vascular endothelial growth factor A (VEGF-A) promotes venous endothelial cell proliferation, resulting in the formation of leaky vessels around demyelinating lesions in multiple sclerosis and experimental autoimmune encephalomyelitis.
VEGF-A 介导的静脉内皮细胞增殖导致神经炎症期间的新血管生成
多发性硬化症(MS)和实验性自身免疫性脑脊髓炎(EAE)的脱髓鞘急慢性病变中存在新形成的渗漏血管和血脑屏障(BBB)损伤。然而,导致这些新血管渗漏的内皮细胞亚型和信号通路尚不清楚。在这里,我们利用单细胞转录谱分析和体内验证研究表明,在雌性成年小鼠的急性和慢性 EAE 病变中,静脉内皮细胞表达新血管生成基因特征,并显示出增殖增加,导致静脉扩大和静脉覆盖率升高。这些变化与血管内皮生长因子 A(VEGF-A)信号的上调有关。我们还证实了新血管生成标记物在急性和慢性人类多发性硬化病变中的表达增加。用 VEGF-A 阻断抗体治疗可减少 EAE 雌性成年小鼠的新血管生成转录组特征和血管增殖,但不能恢复 BBB 功能或改善 EAE 病理。我们的数据证明,静脉内皮细胞有助于脱髓鞘神经炎病症中的新血管生成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


