The role of the haematopoietic stem cell niche in development and ageing
IF 90.2
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
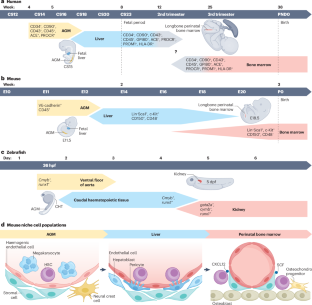
Blood production depends on rare haematopoietic stem cells (HSCs) and haematopoietic stem and progenitor cells (HSPCs) that ultimately take up residence in the bone marrow during development. HSPCs and HSCs are subject to extrinsic regulation by the bone marrow microenvironment, or niche. Studying the interactions between HSCs and their niche is critical for improving ex vivo culturing conditions and genetic manipulation of HSCs, which is pivotal for improving autologous HSC therapies and transplantations. Additionally, understanding how the complex molecular network in the bone marrow is altered during ageing is paramount for developing novel therapeutics for ageing-related haematopoietic disorders. HSCs are unique amongst stem and progenitor cell pools in that they engage with multiple physically distinct niches during their ontogeny. HSCs are specified from haemogenic endothelium in the aorta, migrate to the fetal liver and, ultimately, colonize their final niche in the bone marrow. Recent studies employing single-cell transcriptomics and microscopy have identified novel cellular interactions that govern HSC specification and engagement with their niches throughout ontogeny. New lineage-tracing models and microscopy tools have raised questions about the numbers of HSCs specified, as well as the functional consequences of HSCs interacting with each developmental niche. Advances have also been made in understanding how these niches are modified and perturbed during ageing, and the role of these altered interactions in haematopoietic diseases. In this Review, we discuss these new findings and highlight the questions that remain to be explored. Blood production depends on haematopoietic stem cells (HSCs) and progenitor cells, which are regulated by their microenvironment or niche. New lineage-tracing models and microscopy tools are increasing the understanding of HSC specification and function, and how stem cell–niche interactions are perturbed during ageing.

造血干细胞龛在发育和衰老中的作用
造血依赖于稀有的造血干细胞(HSCs)和造血干细胞及祖细胞(HSPCs),这些细胞在发育过程中最终会进入骨髓。造血干细胞和造血干细胞受到骨髓微环境或骨髓龛的外在调节。研究造血干细胞及其生态位之间的相互作用对于改善造血干细胞的体内外培养条件和遗传操作至关重要,这对于改善自体造血干细胞疗法和移植至关重要。此外,了解骨髓中复杂的分子网络如何在衰老过程中发生变化,对于开发治疗衰老相关造血疾病的新型疗法至关重要。造血干细胞在干细胞和祖细胞库中是独一无二的,因为它们在发育过程中会与多个物理上不同的龛位接触。造血干细胞从主动脉的造血内皮细胞中分化出来,迁移到胎儿肝脏,并最终在骨髓中定植。最近采用单细胞转录组学和显微镜技术进行的研究发现了一些新的细胞相互作用,这些相互作用支配着造血干细胞的分化以及在整个发育过程中与其龛位的接触。新的血缘追踪模型和显微镜工具提出了有关造血干细胞的数量以及造血干细胞与每个发育龛位相互作用的功能性后果的问题。在了解这些龛位如何在衰老过程中发生改变和干扰以及这些改变的相互作用在造血疾病中的作用方面也取得了进展。在本综述中,我们将讨论这些新发现,并强调仍有待探索的问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
173.60
自引率
0.50%
发文量
118
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Molecular Cell Biology is a prestigious journal that aims to be the primary source of reviews and commentaries for the scientific communities it serves. The journal strives to publish articles that are authoritative, accessible, and enriched with easily understandable figures, tables, and other display items. The goal is to provide an unparalleled service to authors, referees, and readers, and the journal works diligently to maximize the usefulness and impact of each article. Nature Reviews Molecular Cell Biology publishes a variety of article types, including Reviews, Perspectives, Comments, and Research Highlights, all of which are relevant to molecular and cell biologists. The journal's broad scope ensures that the articles it publishes reach the widest possible audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


