Oligodendroglial fatty acid metabolism as a central nervous system energy reserve
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
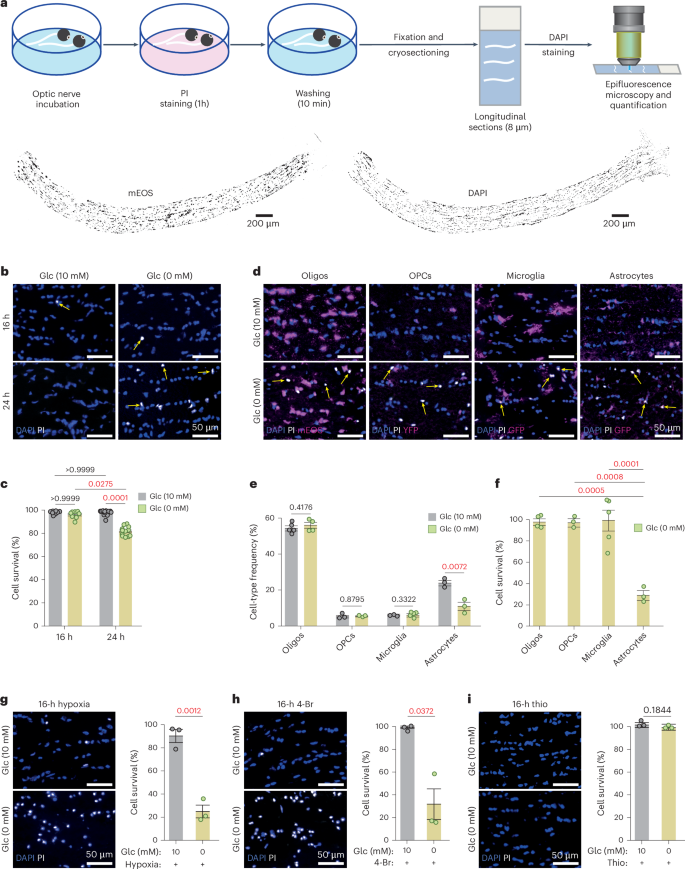
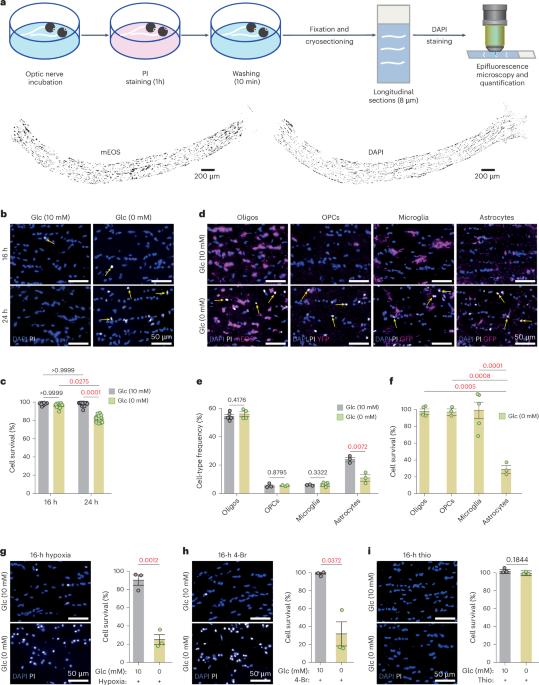
Brain function requires a constant supply of glucose. However, the brain has no known energy stores, except for glycogen granules in astrocytes. In the present study, we report that continuous oligodendroglial lipid metabolism provides an energy reserve in white matter tracts. In the isolated optic nerve from young adult mice of both sexes, oligodendrocytes survive glucose deprivation better than astrocytes. Under low glucose, both axonal ATP levels and action potentials become dependent on fatty acid β-oxidation. Importantly, ongoing oligodendroglial lipid degradation feeds rapidly into white matter energy metabolism. Although not supporting high-frequency spiking, fatty acid β-oxidation in mitochondria and oligodendroglial peroxisomes protects axons from conduction blocks when glucose is limiting. Disruption of the glucose transporter GLUT1 expression in oligodendrocytes of adult mice perturbs myelin homeostasis in vivo and causes gradual demyelination without behavioral signs. This further suggests that the imbalance of myelin synthesis and degradation can underlie myelin thinning in aging and disease. Brain functions require a constant supply of glucose. However, the brain energy stores are unclear. Here, the authors show that oligodendroglial fatty acid metabolism can be an energy reserve for white matter axons, supporting their function.
作为中枢神经系统能量储备的少突胶质细胞脂肪酸代谢
大脑功能需要葡萄糖的持续供应。然而,除了星形胶质细胞中的糖原颗粒外,大脑没有已知的能量储备。在本研究中,我们报告了持续的少突胶质细胞脂质代谢为白质束提供了能量储备。在离体的成年雌雄小鼠视神经中,少突胶质细胞比星形胶质细胞更能在葡萄糖剥夺中存活。在低葡萄糖条件下,轴突的 ATP 水平和动作电位都依赖于脂肪酸的β-氧化。重要的是,持续的少突胶质细胞脂质降解会迅速影响白质的能量代谢。线粒体和少突胶质细胞过氧体中的脂肪酸β-氧化虽然不能支持高频尖峰脉冲,但却能在葡萄糖受限时保护轴突免受传导阻滞。成年小鼠少突胶质细胞中葡萄糖转运体 GLUT1 的表达中断会扰乱体内髓鞘的稳态,并导致逐渐脱髓鞘而无行为症状。这进一步表明,髓鞘合成和降解的失衡可能是衰老和疾病导致髓鞘变薄的原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


