Vonoprazan Dual or Triple Therapy Versus Bismuth-Quadruple Therapy as First-Line Therapy for Helicobacter pylori Infection: A Three-Arm, Randomized Clinical Trial
Abstract
Background
We compared efficacy of vonoprazan-dual or triple therapies and bismuth-quadruple therapy for treatment-naive Helicobacter pylori (HP) infection in Southern China, where primary resistance rates of clarithromycin and levofloxacin are >30%.
Methods
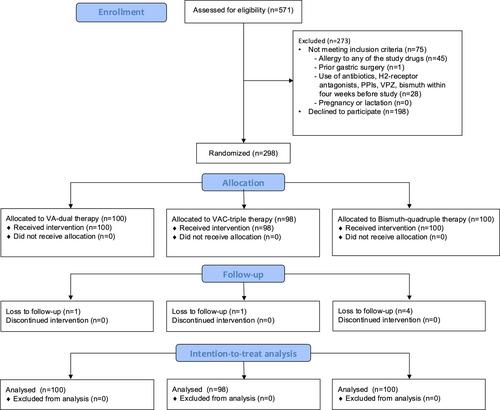
This was an investigator-initiated, three-arm, randomized clinical trial in Southern China. Between March 2022 and August 2023, treatment-naïve HP-infected adults were randomly assigned to receive one of three 14-day regimens (1:1:1 ratio): vonoprazan-dual (VA-dual; vonoprazan 20 mg twice daily and amoxicillin 1 g thrice daily), vonoprazan-triple (VAC-triple; vonoprazan 20 mg/amoxicillin 1 g/clarithromycin 500 mg twice daily), or bismuth-quadruple therapy containing bismuth, esomeprazole, tetracycline, and metronidazole. Primary outcome was noninferiority in HP eradication, evaluated by UBT 4–6 weeks post-treatment by intention-to-treat (ITT) and per-protocol (PP) analysis (based on subjects who completed 14-day treatment and rechecked UBT). Bonferroni-adjusted p-value of <0.017 was used to determine statistical significance.
Results
A total of 298 subjects (mean age: 35.7 ± 8.4 years; male: 134 [45.0%]; VC-dual: 100, VAC-triple: 98, bismuth-quadruple: 100) were enrolled, and 292 (98.0%) had UBT rechecked. ITT analysis showed that both VA-dual (eradication rate of 96.0%) and VAC-triple therapies (95.9%) were noninferior to bismuth-quadruple therapy (92.0%) (difference: 4.0%, 95% CI: −2.9% to 11.5%, p < 0.001; and 3.9%, 95% CI: −3.1% to 11.5%, p < 0.001, respectively). PP analysis also revealed noninferiority (96.7% or 96.7% vs. 97.4%, with difference: −2.9% and −2.9%, p = 0.009 and 0.010, respectively). The frequency of adverse events was 39.0%, 56.1%, and 71.0% in VA-dual, VAC-triple, and bismuth-quadruple therapies, respectively.
Conclusions
VA-dual and VA-triple therapies are highly effective and noninferior to bismuth-quadruple therapy in Southern China. Given the lower adverse effects and fewer antibiotic use, VA-dual therapy is the preferred first-line treatment for HP infection.
Trial Registration
Chinese Clinical Trial Registry (No. ChiCTR2200056375). Registered on February 4, 2022, https://www.chictr.org.cn/showproj.aspx?proj=14131.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


