Effect of N-heterocyclic carbenes-Pt catalytic system and crosslinking networks on the pyrolytic behavior of liquid silicone rubber
Abstract
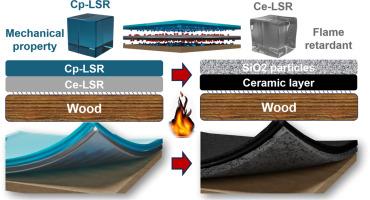
Liquid silicone rubber (LSR) exhibits excellent thermal stability and has been selected for use in a variety of applications where thermal stability, chemical resistance and fire-retardant are required. The enhancement of the organic-to-inorganic conversion of LSR to improve their flame-retardant properties represents a significant area of research. The thermal stability of the platinum catalysts and the crosslinked network structure of the LSR have a considerable influence on the organic-to-organic conversion behavior of LSR. The present study demonstrates the efficacy of N-heterocyclic carbene (NHC) ligand-modified Karstedt's catalysts as catalysts for the curing of LSR by hydrosilylation at room temperature and for the organic-to-inorganic conversion of LSR at elevated temperatures. The catalyst was employed in the preparation of three LSRs with varying network structures, utilizing four polysiloxanes with differing degrees of functionality. The pyrolytic behavior and organic-to-organic conversion rate of these LSRs were investigated using a thermogravimetric analyzer (TG) coupled with a Fourier transform infrared spectrometer (FTIR). The findings indicated that LSRs with the highest crosslink density exhibited the highest organic-to-inorganic conversion rate; however, they demonstrated the lowest fire-resistance. The anomalous behavior has been subjected to further analysis with respect to the mechanical properties of the LSRs and the characteristics of their network structure. LSR coatings with enhanced hardness and fire-resistance are then produced by combining the advantageous properties of both LSRs in a layer-by-layer (LBL) assembly.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


