Induced pluripotent stem cell-derived neural stem cells promote bone formation in mice with calvarial defects
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Nerve-derived factors have attracted attention in bone regeneration therapy due to their ability to promote bone regeneration and nerve innervation. Mesenchymal stem cells transported to target sites promote osteogenesis. However, there are few reports on the effects of neural stem cells on bone regeneration. Therefore, the aim of this study was to investigate the role of neural stem cells in osteogenesis. Here, embryoid bodies (EB) or primary neurospheres (1NS) were generated using mouse induced pluripotent stem cells (iPS cells), which were then seeded onto gelatin (Gel) sponges. The seeded Gel sponges were then transplanted into mouse calvarial bone defects. We noted that 1NS-seeded Gel promoted bone regeneration and the presence of tartrate-resistant acid phosphatase (TRAP)-positive cells, whereas the EB-seeded Gel did not. RNA-sequencing of the 1NS-seeded and EB seeded Gels showed an upregulation of the transforming growth factor (TGF)-β signaling pathway in the 1NS-seeded Gel group. Immunostaining confirmed the presence of Id3 positive cells in mice with bone defects treated with the 1NS-seeded Gel. These findings suggest that the transplantation of neural stem cells may contribute to the promotion of bone regeneration.
Statement of significance
This study aimed to investigate whether neural stem cells, when seeded in Gel sponges, promoted bone regeneration. It has been well documented that bone is tightly linked with the nervous systems. Bioscaffolds comprising factors that promote innervation and bone regeneration have been investigated for use in bone therapy. However, there is limited research on the use of neural stem cells for promoting bone formation. To assess this relationship, we conducted both in vivo and in vitro assays to determine whether neural stem cells promoted bone formation. We noted that 1NS-seeded Gel sponges promoted bone formation significantly in mice with calvarial defects after 4 weeks. This study provides a novel approach of neural stem cells for bone therapy.
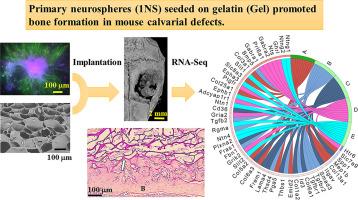
诱导多能干细胞衍生的神经干细胞可促进腓骨缺损小鼠的骨形成。
神经源因子具有促进骨再生和神经支配的能力,因此在骨再生疗法中备受关注。间充质干细胞运输到目标部位可促进骨生成。然而,有关神经干细胞对骨再生影响的报道却很少。因此,本研究旨在探讨神经干细胞在骨生成中的作用。本研究利用小鼠诱导多能干细胞(iPS细胞)生成类胚体(EB)或初级神经球(1NS),然后将其播种到明胶(Gel)海绵上。然后将播种的明胶海绵移植到小鼠腓骨缺损处。我们注意到 1NS 种子凝胶促进了骨再生和 TRAP 阳性细胞的出现,而 EB 种子凝胶则没有。对 1NS 种子凝胶和 EB 种子凝胶进行的 RNA 序列分析表明,在 1NS 种子凝胶组中,TGF-β 信号通路上调。免疫染色证实,使用1NS种子凝胶治疗骨缺损的小鼠体内存在Id3阳性细胞。这些发现表明,移植神经干细胞可能有助于促进骨再生。意义说明:本研究旨在探讨神经干细胞在明胶海绵中播种是否能促进骨再生。有大量文献表明,骨骼与神经系统密切相关。研究人员已将含有促进神经支配和骨再生因子的生物支架用于骨治疗。然而,有关使用神经干细胞促进骨形成的研究却很有限。为了评估这种关系,我们进行了体内和体外试验,以确定神经干细胞是否促进骨形成。我们注意到,原代神经球播种明胶海绵在四周后能显著促进腓骨缺损小鼠的骨形成。这项研究提供了神经干细胞用于骨治疗的新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


