Integrative proteogenomic profiling of high-risk prostate cancer samples from Chinese patients indicates metabolic vulnerabilities and diagnostic biomarkers
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
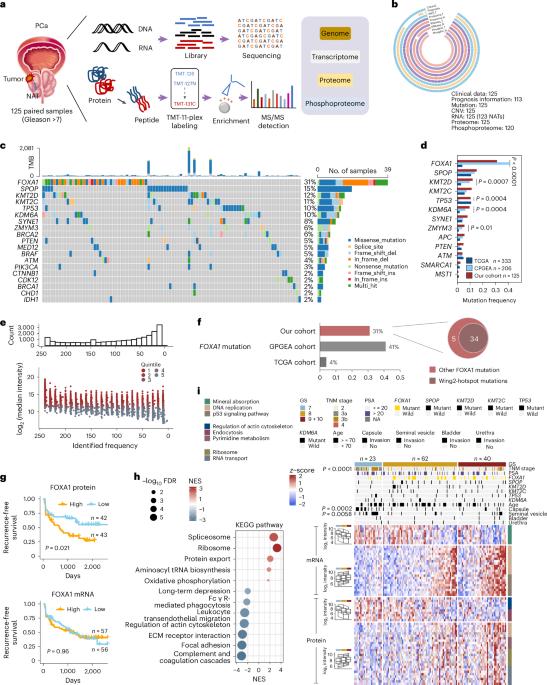
Prostate cancer (PCa) exhibits significant geoethnic disparities as reflected by distinct variations in the cancer genome and disease progression. Here, we perform a comprehensive proteogenomic characterization of localized high-risk PCa utilizing paired tumors and nearby tissues from 125 Chinese male patients, with the primary objectives of identifying potential biomarkers, unraveling critical oncogenic events and delineating molecular subtypes with poor prognosis. Our integrated analysis highlights the utility of GOLM1 as a noninvasive serum biomarker. Phosphoproteomics analysis reveals the crucial role of Ser331 phosphorylation on FOXA1 in regulating FOXA1-AR-dependent cistrome. Notably, our proteomic profiling identifies three distinct subtypes, with metabolic immune-desert tumors (S-III) emerging as a particularly aggressive subtype linked to poor prognosis and BCAT2 catabolism-driven PCa progression. In summary, our study provides a comprehensive resource detailing the unique proteomic and phosphoproteomic characteristics of PCa molecular pathogenesis and offering valuable insights for the development of diagnostic and therapeutic strategies. Dong et al. present an integrative proteogenomic analysis of high-risk prostate cancer samples from a cohort of Chinese patients and highlight potential therapeutic vulnerabilities and diagnostic markers.
对中国高危前列腺癌样本的综合蛋白质基因组分析表明了代谢脆弱性和诊断生物标志物。
前列腺癌(PCa)表现出明显的地缘种族差异,这反映在癌症基因组和疾病进展的不同变化上。在这里,我们利用来自 125 名中国男性患者的配对肿瘤和邻近组织,对局部高危 PCa 进行了全面的蛋白质基因组学鉴定,主要目的是确定潜在的生物标志物、揭示关键的致癌事件以及划分预后不良的分子亚型。我们的综合分析凸显了 GOLM1 作为非侵入性血清生物标记物的实用性。磷酸化蛋白质组学分析揭示了 FOXA1 上 Ser331 磷酸化在调控 FOXA1-AR 依赖性细胞色素中的关键作用。值得注意的是,我们的蛋白质组学分析确定了三种不同的亚型,其中代谢免疫凋亡肿瘤(S-III)是一种侵袭性特别强的亚型,与预后不良和 BCAT2 分解代谢驱动的 PCa 进展有关。总之,我们的研究提供了一个全面的资源,详细说明了 PCa 分子发病机制的独特蛋白质组和磷酸化蛋白质组特征,为诊断和治疗策略的开发提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


