Pharmacokinetic and Preclinical Safety Studies of Endolysin-Based Therapeutic for Intravenous Administration
IF 4.9
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2024-09-05
DOI:10.1016/j.ijantimicag.2024.107328
引用次数: 0
Abstract
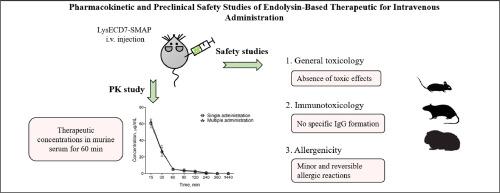
Pharmacokinetics and safety studies of innovative drugs is an essential part of drug development process. Previously we have developed a novel drug for intravenous administration (lyophilizate) containing modified endolysin LysECD7-SMAP that showed notable antibacterial effect in different animal models of systemic infections. Here we present data on pharmacokinetics of endolysin in mice after single and multiple injections. Time-concentration curves were obtained, and pharmacokinetic parameters for preparation (C0, kel t1/2, AUC0–∞, MRT, ClT, Vss) were calculated. It was shown that although endolysin is rather short-lived in blood serum (t1/2 = 12.5 min), the therapeutic concentrations of LysECD7-SMAP (in degraded and non-degraded form) were detected for 60 minutes after injection that is sufficient for antibacterial effect. Based on the obtained data, it was proposed that endolysin distributes presumably in murine blood, degrades in blood and liver, and is eliminated via glomerular filtration. Safety profile of the preparation relating to general toxicity, immunotoxicity and allergenicity was assessed in rodents. It was demonstrated that LysECD7-SMAP in potential therapeutic (12.5 mg/kg), 10-fold (125 mg/kg) and 40-fold (500 mg/kg) doses showed no signs of intoxication and significant abnormalities after single and repeated i.v. administrations, preparation was non-immunogenic and induced minor and reversible allergic reaction in animals.
基于内溶菌素的静脉注射治疗药物的药代动力学和临床前安全性研究
创新药物的药代动力学和安全性研究是药物开发过程的重要组成部分。此前,我们开发了一种新型静脉注射药物(冻干溶液),其中含有改良的内切酶 LysECD7-SMAP,在不同的全身感染动物模型中显示出显著的抗菌效果。在此,我们介绍了内溶菌素在小鼠体内单次和多次注射后的药代动力学数据。我们获得了时间-浓度曲线,并计算了制剂的药代动力学参数(C0、kel t1/2、AUC0-∞、MRT、ClT、Vss)。结果表明,虽然内溶血素在血清中的存活时间很短(t1/2 = 12.5 分钟),但在注射后 60 分钟内仍能检测到 LysECD7-SMAP(降解和非降解形式)的治疗浓度,这足以产生抗菌效果。根据获得的数据,推测内溶血素可能分布在小鼠血液中,在血液和肝脏中降解,并通过肾小球滤过排出体外。在啮齿动物身上评估了制剂的一般毒性、免疫毒性和过敏性等安全性。结果表明,LysECD7-SMAP 的潜在治疗剂量(12.5 毫克/千克)、10 倍剂量(125 毫克/千克)和 40 倍剂量(500 毫克/千克)在单次和多次静脉注射后不会出现中毒症状和明显异常。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


