Accurate prediction of antimicrobial resistance and genetic marker of Staphylococcus aureus clinical isolates using MALDI-TOF MS and machine learning – across DRIAMS and Taiwan database
IF 4.9
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2024-09-06
DOI:10.1016/j.ijantimicag.2024.107329
引用次数: 0
Abstract
Background
The use of matrix-assisted laser desorption/ionisation-time-of-flight mass spectra (MALDI-TOF MS) with machine learning (ML) has been explored for predicting antimicrobial resistance. This study evaluates the effectiveness of MALDI-TOF MS paired with various ML classifiers and establishes optimal models for predicting antimicrobial resistance and the presence of mecA gene among Staphylococcus aureus.
Materials and Methods
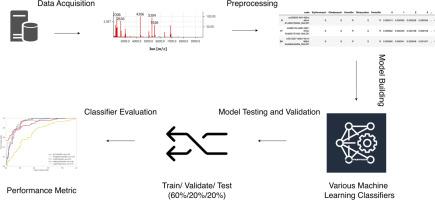
Antimicrobial resistance against tier 1 antibiotics and MALDI-TOF MS of S. aureus were analysed using data from the Database of Resistance against Antimicrobials with MALDI-TOF Mass Spectrometry (DRIAMS) and one medical centre (CS database). Five ML classifiers were used to analyse performance metrics. The Shapley value quantified the predictive contribution of individual features.
Results
The LightGBM demonstrated superior performance in predicting antimicrobial resistance for most tier 1 antibiotics among oxacillin-resistant S. aureus (ORSA) compared with all S. aureus and oxacillin-susceptible S. aureus (OSSA) in both databases. In DRIAMS, Multilayer Perceptron (MLP) was associated with excellent predictive performance, expressed as accuracy/AUROC/AUPR, for clindamycin (0.74/0.81/0.90), tetracycline (0.86/0.87/0.94), and trimethoprim-sulfamethoxazole (0.95/0.72/0.97). In the CS database, Ada and Light Gradient Boosting Machine (LightGBM) showed excellent performance for erythromycin (0.97/0.92/0.86) and tetracycline (0.68/0.79/0.86). Mass-to-charge ratio (m/z) features of 2411–2414 and 2429–2432 correlated with clindamycin resistance, whereas 5033–5036 was linked to erythromycin resistance in DRIAMS. In the CS database, overlapping features of 2423–2426, 4496–4499, and 3764–3767 simultaneously predicted the presence of mecA and oxacillin resistance.
Conclusion
The predictive performance of antimicrobial resistance against S. aureus using MALDI-TOF MS depends on database characteristics and the ML algorithm selected. Specific and overlapping mass spectra features are excellent predictive markers for mecA and specific antimicrobial resistance.
利用 MALDI-TOF MS 和机器学习准确预测金黄色葡萄球菌临床分离株的抗菌药耐药性和遗传标记 - 跨 DRIAMS 和台湾数据库。
背景:基质辅助激光解吸/电离飞行时间质谱(MALDI-TOF MS)与机器学习(ML)的结合被用于预测抗菌药耐药性。本研究评估了 MALDI-TOF MS 与各种 ML 分类器配对的有效性,并建立了预测金黄色葡萄球菌抗菌药耐药性和 mecA 基因存在情况的最佳模型:利用MALDI-TOF质谱法抗菌药耐药性数据库(DRIAMS)和一个医疗中心(CS数据库)的数据,分析了金黄色葡萄球菌对一级抗生素和MALDI-TOF MS的抗菌药耐药性。五种 ML 分类器用于分析性能指标。Shapley 值量化了单个特征的预测贡献:结果:在这两个数据库中,LightGBM 在预测耐奥沙西林金黄色葡萄球菌(ORSA)对大多数 1 级抗生素的抗菌性方面表现优于所有金黄色葡萄球菌和对奥沙西林敏感的金黄色葡萄球菌(OSSA)。在 DRIAMS 数据库中,MLP 对克林霉素(0.74/0.81/0.90)、四环素(0.86/0.87/0.94)和三甲双氨-磺胺甲噁唑(0.95/0.72/0.97)具有出色的预测性能,以准确率/AUROC/AUPR 表示。在 CS 数据库中,Ada 和 LightGBM 分别对红霉素(0.97/0.92/0.86)和四环素(0.68/0.79/0.86)保持了卓越的性能。在 DRIAMS 中,2,411-2,414 和 2,429-2,432 的质荷比(m/z)特征与克林霉素耐药性相关,而 5,033-5,036 与红霉素耐药性相关。在 CS 数据库中,2,423-2,426、4,496-4,499 和 3,764-3,767 个重叠特征同时预测了 mecA 的存在和对奥沙西林的耐药性:结论:使用 MALDI-TOF MS 预测金黄色葡萄球菌抗菌药耐药性的性能取决于数据库特征和所选的 ML 算法。特定和重叠的 MS 特征是预测 mecA 和特定抗菌素耐药性的极佳标记。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


