Thr4 phosphorylation on RNA Pol II occurs at early transcription regulating 3′-end processing
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
RNA polymerase II relies on a repetitive sequence domain (YSPTSPS) within its largest subunit to orchestrate transcription. While phosphorylation on serine-2/serine-5 of the carboxyl-terminal heptad repeats is well established, threonine-4’s role remains enigmatic. Paradoxically, threonine-4 phosphorylation was only detected after transcription end sites despite functionally implicated in pausing, elongation, termination, and messenger RNA processing. Our investigation revealed that threonine-4 phosphorylation detection was obstructed by flanking serine-5 phosphorylation at the onset of transcription, which can be removed selectively. Subsequent proteomic analyses identified many proteins recruited to transcription via threonine-4 phosphorylation, which previously were attributed to serine-2. Loss of threonine-4 phosphorylation greatly reduces serine-2 phosphorylation, revealing a cross-talk between the two marks. Last, the function analysis of the threonine-4 phosphorylation highlighted its role in alternative 3′-end processing within pro-proliferative genes. Our findings unveil the true genomic location of this evolutionarily conserved phosphorylation mark and prompt a reassessment of functional assignments of the carboxyl-terminal domain.
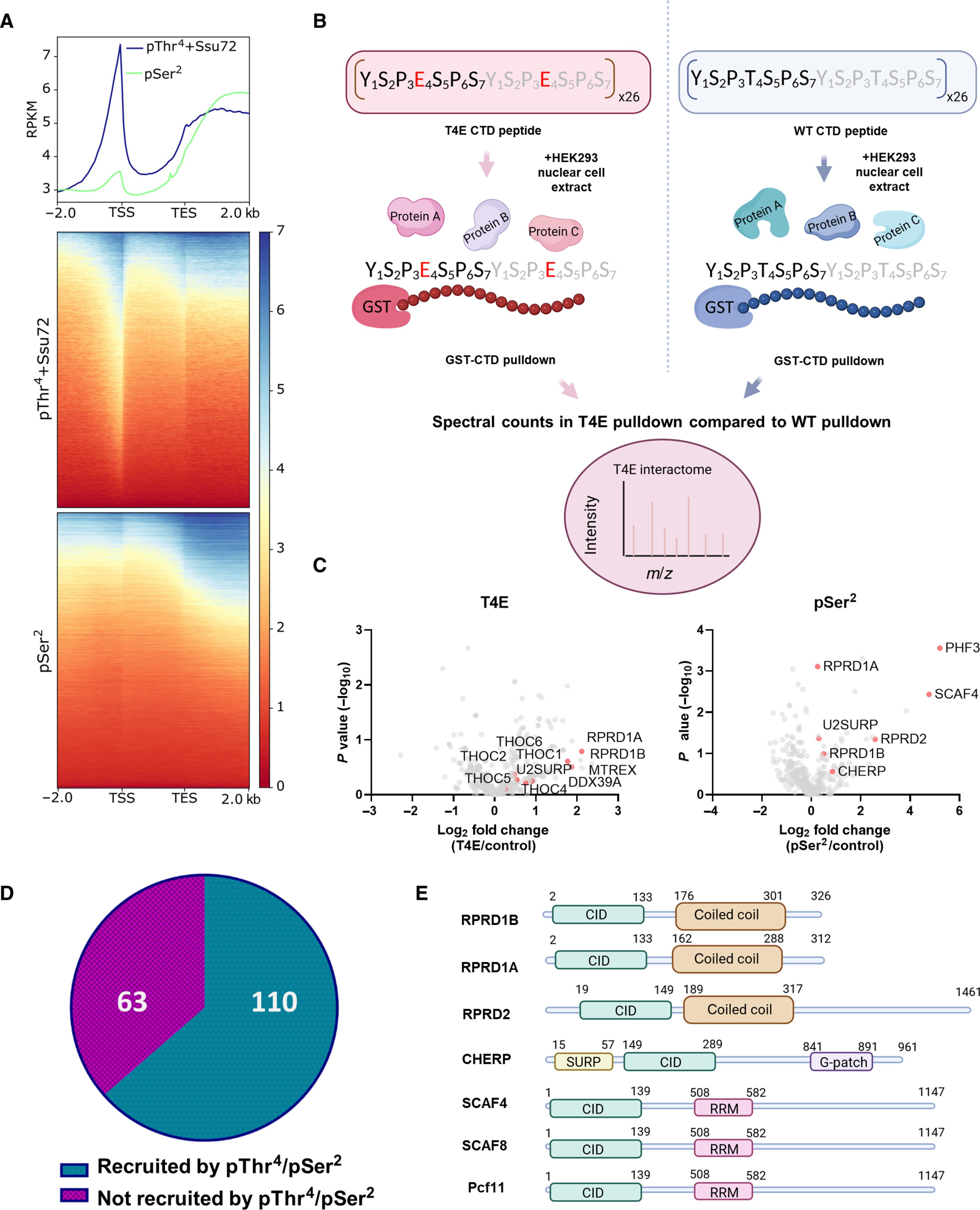
RNA Pol II 上的 Thr4 磷酸化发生在调节 3'-end 处理的转录早期。
RNA 聚合酶 II 依靠其最大亚基中的重复序列结构域(YSPTSPS)来协调转录。虽然羧基末端七联重复序列的丝氨酸-2/丝氨酸-5的磷酸化作用已被证实,但苏氨酸-4的作用仍是个谜。奇怪的是,尽管苏氨酸-4 在功能上与转录暂停、延长、终止和信使 RNA 处理有关,但只有在转录终止位点后才检测到苏氨酸-4 磷酸化。我们的研究发现,苏氨酸-4 磷酸化的检测受到转录开始时侧翼丝氨酸-5 磷酸化的阻碍,而丝氨酸-5 磷酸化可以被选择性地去除。随后的蛋白质组分析发现了许多通过苏氨酸-4 磷酸化被转录所招募的蛋白质,而这些蛋白质之前被归因于丝氨酸-2。苏氨酸-4 磷酸化的缺失大大降低了丝氨酸-2 磷酸化,揭示了这两种标记之间的交叉作用。最后,苏氨酸-4 磷酸化的功能分析强调了它在促增殖基因的替代 3'- 末端处理中的作用。我们的发现揭示了这一进化保守的磷酸化标记的真正基因组位置,并促使我们重新评估羧基末端结构域的功能分配。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


