A high-throughput workflow to analyze sequence-conformation relationships and explore hydrophobic patterning in disordered peptoids
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Understanding how a macromolecule’s primary sequence governs its conformational landscape is crucial for elucidating its function, yet these design principles are still emerging for macromolecules with intrinsic disorder. Herein, we introduce a high-throughput workflow that implements a practical colorimetric conformational assay, introduces a semi-automated sequencing protocol using matrix-assisted laser desorption/ionization and tandem mass spectrometry (MALDI-MS/MS), and develops a generalizable sequence-structure algorithm. Using a model system of 20mer peptidomimetics containing polar glycine and hydrophobic N-butylglycine residues, we identified nine classifications of conformational disorder and isolated 122 unique sequences across varied compositions and conformations. Conformational distributions of three compositionally identical library sequences were corroborated through atomistic simulations and ion mobility spectrometry coupled with liquid chromatography. A data-driven strategy was developed using existing sequence variables and data-derived “motifs” to inform a machine-learning algorithm toward conformation prediction. This multifaceted approach enhances our understanding of sequence-conformation relationships and offers a powerful tool for accelerating the discovery of materials with conformational control.
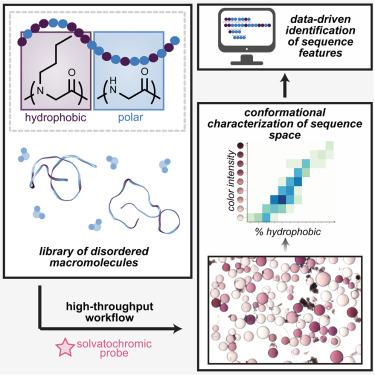
分析序列构象关系和探索无序蛋白胨疏水模式的高通量工作流程
了解大分子的主序列如何支配其构象格局对于阐明其功能至关重要,然而这些设计原则对于具有内在无序性的大分子来说仍是崭露头角。在本文中,我们介绍了一种高通量工作流程,它实现了一种实用的比色构象测定法,介绍了一种使用基质辅助激光解吸/电离和串联质谱(MALDI-MS/MS)的半自动测序方案,并开发了一种可通用的序列结构算法。利用含有极性甘氨酸和疏水性 N-丁基甘氨酸残基的 20 聚合物拟肽模型系统,我们确定了九种构象紊乱分类,并分离出 122 个不同组成和构象的独特序列。通过原子模拟和离子迁移谱-液相色谱法,我们证实了三个成分相同的文库序列的构象分布。利用现有序列变量和数据衍生的 "图案 "开发了一种数据驱动策略,为构象预测的机器学习算法提供信息。这种多方面的方法增强了我们对序列-构象关系的理解,为加速发现具有构象控制能力的材料提供了强有力的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


