Exocyst stimulates multiple steps of exocytic SNARE complex assembly and vesicle fusion
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Exocyst is a large multisubunit tethering complex essential for targeting and fusion of secretory vesicles in eukaryotic cells. Although the assembled exocyst complex has been proposed to tether vesicles to the plasma membrane and activate soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) for membrane fusion, the key biochemical steps that exocyst stimulates in SNARE-mediated fusion are undetermined. Here we use a combination of single-molecule and bulk fluorescence assays to investigate the roles of purified octameric yeast exocyst complexes in a reconstituted yeast exocytic SNARE assembly and vesicle fusion system. Exocyst had stimulatory roles in multiple distinct steps ranging from SNARE protein activation to binary and ternary complex assembly. Importantly, exocyst had a downstream role in driving membrane fusion and full content mixing of vesicle lumens. Our data suggest that exocyst provides extensive chaperoning functions across the entire process of SNARE complex assembly and fusion, thereby governing exocytosis at multiple steps. Exocytosis of secretory vesicles is required for cellular growth, cellular division and cell–cell communication. Lee et al. reveal that the exocyst tethering complex has stimulatory roles in exocytic soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex assembly and SNARE-mediated vesicle fusion.
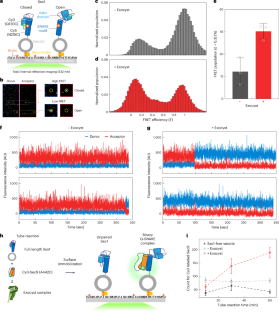

外囊刺激外细胞 SNARE 复合物组装和囊泡融合的多个步骤
外囊泡是一种大型多亚基拴系复合体,对于真核细胞中分泌囊泡的靶向和融合至关重要。尽管有人提出组装的外囊复合体可将囊泡拴系到质膜上并激活可溶性 N-乙基马来酰亚胺敏感因子附着蛋白受体(SNARE)以实现膜融合,但外囊刺激 SNARE 介导的融合的关键生化步骤尚未确定。在这里,我们结合使用单分子和大量荧光测定法,研究了纯化的八聚体酵母外囊复合物在重组的酵母外细胞 SNARE 组装和囊泡融合系统中的作用。外囊在从SNARE蛋白活化到二元和三元复合物组装的多个不同步骤中都起着刺激作用。重要的是,外囊在驱动膜融合和囊泡腔的全含量混合方面起着下游作用。我们的数据表明,外囊在整个SNARE复合物组装和融合过程中提供了广泛的伴侣功能,从而在多个步骤上控制了外吞作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


