Primary succession of Bifidobacteria drives pathogen resistance in neonatal microbiota assembly
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
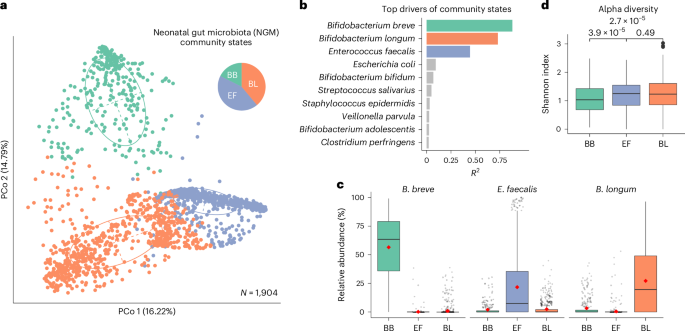
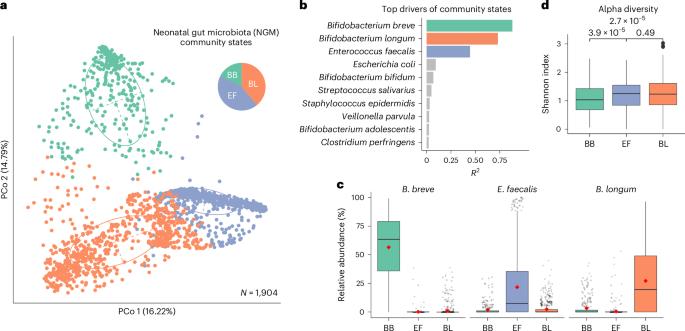
Human microbiota assembly commences at birth, seeded by both maternal and environmental microorganisms. Ecological theory postulates that primary colonizers dictate microbial community assembly outcomes, yet such microbial priority effects in the human gut remain underexplored. Here using longitudinal faecal metagenomics, we characterized neonatal microbiota assembly for a cohort of 1,288 neonates from the UK. We show that the pioneering neonatal gut microbiota can be stratified into one of three distinct community states, each dominated by a single microbial species and influenced by clinical and host factors, such as maternal age, ethnicity and parity. A community state dominated by Enterococcus faecalis displayed stochastic microbiota assembly with persistent high pathogen loads into infancy. In contrast, community states dominated by Bifidobacterium, specifically B. longum and particularly B. breve, exhibited a stable assembly trajectory and long-term pathogen colonization resistance, probably due to strain-specific functional adaptions to a breast milk-rich neonatal diet. Consistent with our human cohort observation, B. breve demonstrated priority effects and conferred pathogen colonization resistance in a germ-free mouse model. Our findings solidify the crucial role of Bifidobacteria as primary colonizers in shaping the microbiota assembly and functions in early life. Primary colonization by microbial communities dominated by Bifidobacteria contribute to stable gut microbiota assembly and long-term pathogen resistance in neonates.
双歧杆菌的初级演替驱动新生儿微生物群的病原体抵抗力
人类微生物群的形成始于出生时,由母体和环境微生物共同播种。生态学理论推测,初级定植者决定了微生物群落的组合结果,但人类肠道中的这种微生物优先效应仍未得到充分探索。在这里,我们利用纵向粪便元基因组学,对英国 1288 名新生儿的微生物群落组合进行了描述。我们的研究表明,新生儿肠道微生物群可分为三种不同的群落状态,每种状态都由单一微生物物种主导,并受临床和宿主因素(如母亲年龄、种族和胎次)的影响。以粪肠球菌为主的群落状态显示出随机的微生物群组合,并在婴儿期持续存在高病原体负荷。相比之下,双歧杆菌(特别是长双歧杆菌,尤其是前双歧杆菌)主导的群落状态则表现出稳定的集结轨迹和长期的抗病原体定植能力,这可能是由于菌株对富含母乳的新生儿饮食的特异性功能适应所致。与我们的人类队列观察结果一致,布氏杆菌在无菌小鼠模型中表现出优先效应并赋予病原体定植抵抗力。我们的研究结果证实了双歧杆菌作为主要定植菌在塑造生命早期微生物群组合和功能方面的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


