Collagen-targeted protein nanomicelles for the imaging of non-alcoholic steatohepatitis
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
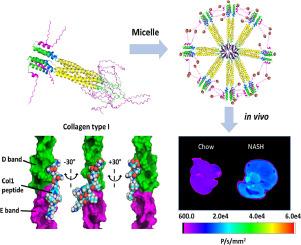
In vivo molecular imaging tools hold immense potential to drive transformative breakthroughs by enabling researchers to visualize cellular and molecular interactions in real-time and/or at high resolution. These advancements will facilitate a deeper understanding of fundamental biological processes and their dysregulation in disease states. Here, we develop and characterize a self-assembling protein nanomicelle called collagen type I binding – thermoresponsive assembled protein (Col1-TRAP) that binds tightly to type I collagen in vitro with nanomolar affinity. For ex vivo visualization, Col1-TRAP is labeled with a near-infrared fluorescent dye (NIR-Col1-TRAP). Both Col1-TRAP and NIR-Col1-TRAP display approximately a 3.8-fold greater binding to type I collagen compared to TRAP when measured by surface plasmon resonance (SPR). We present a proof-of-concept study using NIR-Col1-TRAP to detect fibrotic type I collagen deposition ex vivo in the livers of mice with non-alcoholic steatohepatitis (NASH). We show that NIR-Col1-TRAP demonstrates significantly decreased plasma recirculation time as well as increased liver accumulation in the NASH mice compared to mice without disease over 4 hours. As a result, NIR-Col1-TRAP shows potential as an imaging probe for NASH with in vivo targeting performance after injection in mice.
Statement of significance
Direct molecular imaging of fibrosis in NASH patients enables the diagnosis and monitoring of disease progression with greater specificity and resolution than do elastography-based methods or blood tests. In addition, protein-based imaging probes are more advantageous than alternatives due to their biodegradability and scalable biosynthesis. With the aid of computational modeling, we have designed a self-assembled protein micelle that binds to fibrillar and monomeric collagen in vitro. After the protein was labeled with near-infrared fluorescent dye, we injected the compound into mice fed on a NASH diet. NIR-Col1-TRAP clears from the serum faster in these mice compared to control mice, and accumulates significantly more in fibrotic livers.This work advances the development of targeted protein probes for in vivo fibrosis imaging.
用于非酒精性脂肪性肝炎成像的胶原蛋白靶向蛋白质纳米微孔。
体内分子成像工具使研究人员能够实时和/或高分辨率地观察细胞和分子的相互作用,具有推动变革性突破的巨大潜力。这些进步将有助于深入了解基本生物过程及其在疾病状态下的失调。在这里,我们开发并鉴定了一种名为 I 型胶原蛋白结合-热致伸缩性组装蛋白(Col1-TRAP)的自组装蛋白纳米小体,它能在体外以纳摩尔级的亲和力与 I 型胶原蛋白紧密结合。为了进行体内外可视化,Col1-TRAP 被标记了近红外荧光染料(NIR-Col1-TRAP)。通过表面等离子体共振(SPR)测量,Col1-TRAP 和 NIR-Col1-TRAP 与 I 型胶原蛋白的结合力比 TRAP 高出约 3.8 倍。我们利用 NIR-Col1-TRAP 进行了一项概念验证研究,以检测非酒精性脂肪性肝炎(NASH)小鼠肝脏中的纤维化 I 型胶原沉积。我们的研究表明,与未患病的小鼠相比,NIR-Col1-TRAP 在 NASH 小鼠体内 4 小时的血浆再循环时间明显缩短,肝脏积聚增加。因此,NIR-Col1-TRAP 显示出作为 NASH 的成像探针的潜力,在小鼠注射后具有体内靶向性能。意义声明::与基于弹性成像的方法或血液检测相比,NASH 患者纤维化的直接分子成像能够以更高的特异性和分辨率诊断和监测疾病的进展。此外,基于蛋白质的成像探针因其生物可降解性和可扩展的生物合成而比其他探针更具优势。借助计算模型,我们设计了一种自组装蛋白胶束,可在体外与纤维状和单体胶原蛋白结合。在用近红外荧光染料标记该蛋白后,我们将该化合物注射到以 NASH 为食的小鼠体内。与对照组小鼠相比,这些小鼠体内的蛋白质从血清中清除的速度更快,在纤维化肝脏中的累积量明显增加。这项工作推动了体内纤维化成像靶向蛋白探针的开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


