Precise tracking of nanoparticles in plant roots
IF 13.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
One of the foremost challenges in nanobiotechnology is obtaining direct evidence of nanoparticles’ absorption and internalization in plants. Although confocal laser scanning microscopy (CLSM) or transmission electron microscopy (TEM) are currently the most commonly used tools to characterize nanoparticles in plants, subjectivity of researchers, incorrect sample handling, inevitable fluorescence leakage and limitations of imaging instruments lead to false positives and non-reproducibility of experimental results. This protocol provides an easy-to-operate dual-step method, combining CLSM for macroscopic tissue examination and TEM for cellular-level analysis, to effectively trace single particles in plant roots with accuracy and precision. In addition, we also provide detailed methods for processing plant materials before imaging, including cleaning, and staining, to maximize the accuracy and reliability of imaging. This protocol involves currently commonly used nanomaterial types, such as metal-based and doped carbon-based materials, and enables accurate localization of nanoparticles with different sizes at the cell level in Arabidopsis thaliana root samples either through contrast or element mapping analysis. It serves as a valuable reference and benchmark for scholars in plant science, chemistry and environmental studies to understand the interaction between plant roots and nanomaterials and to detect the distribution of nanomaterials in plants. Excluding plant culture time, the protocol can be completed in 4–5 d. This protocol for the precise tracking of nanoparticles in plant roots uses CLSM for macroscopic tissue examination and TEM for cellular-level analysis and provides methodologies for preparing plant materials before imaging.
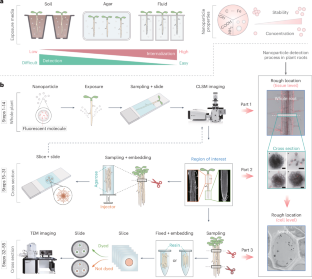

精确跟踪植物根部的纳米粒子。
纳米生物技术的首要挑战之一是获得纳米粒子在植物体内吸收和内化的直接证据。尽管共焦激光扫描显微镜(CLSM)或透射电子显微镜(TEM)是目前表征植物中纳米粒子的最常用工具,但研究人员的主观性、不正确的样品处理、不可避免的荧光泄漏以及成像仪器的局限性导致了假阳性和实验结果的不可再现性。本方案提供了一种易于操作的双步骤方法,将用于宏观组织检查的 CLSM 与用于细胞级分析的 TEM 相结合,可有效、准确地追踪植物根中的单个颗粒。此外,我们还提供了成像前处理植物材料的详细方法,包括清洁和染色,以最大限度地提高成像的准确性和可靠性。该方案涉及目前常用的纳米材料类型,如金属基和掺杂碳基材料,通过对比或元素图谱分析,可在拟南芥根部样本的细胞水平准确定位不同大小的纳米颗粒。它为植物科学、化学和环境研究领域的学者了解植物根系与纳米材料之间的相互作用以及检测纳米材料在植物中的分布提供了宝贵的参考和基准。不包括植物培养时间,该方案可在 4-5 d 内完成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Protocols
生物-生化研究方法
CiteScore
29.10
自引率
0.70%
发文量
128
审稿时长
4 months
期刊介绍:
Nature Protocols focuses on publishing protocols used to address significant biological and biomedical science research questions, including methods grounded in physics and chemistry with practical applications to biological problems. The journal caters to a primary audience of research scientists and, as such, exclusively publishes protocols with research applications. Protocols primarily aimed at influencing patient management and treatment decisions are not featured.
The specific techniques covered encompass a wide range, including but not limited to: Biochemistry, Cell biology, Cell culture, Chemical modification, Computational biology, Developmental biology, Epigenomics, Genetic analysis, Genetic modification, Genomics, Imaging, Immunology, Isolation, purification, and separation, Lipidomics, Metabolomics, Microbiology, Model organisms, Nanotechnology, Neuroscience, Nucleic-acid-based molecular biology, Pharmacology, Plant biology, Protein analysis, Proteomics, Spectroscopy, Structural biology, Synthetic chemistry, Tissue culture, Toxicology, and Virology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


