A benzimidazole-based Cu(ii) complex catalyzed site-selective C–H sulfenylation of imidazo-[1,2-a]pyridines using CS2 as a sulfur source†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A new benzimidazole-based Cu(ii) complex catalyzed site-selective sulfenylation of imidazo[1,2-a]pyridines with benzyl/alkyl/allyl bromides and CS2 at 100 °C in DMF : H2O is reported. The present methodology has been developed for the synthesis of 3-sulfenyl imidazo[1,2-a]pyridines in good yields with a broad substrate scope. In this protocol, CS2, commonly known as a non-polar small molecule bioregulator (SMB), is converted to valuable sulfenylated imidazo[1,2-a]pyridine derivatives. In addition, theoretical investigations along with experimental evidence unfold the insights into the probable mechanistic pathway of site-selective sulfenylation from S,S-dibenzyltrithiocarbonate, which is particularly formed as an intermediate during the reaction.

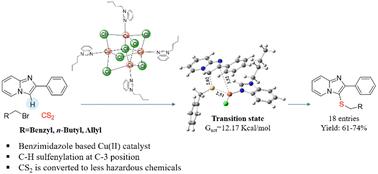
一种苯并咪唑基 Cu(II) 复合物以 CS2 为硫源催化咪唑并[1,2-a]吡啶的位点选择性 C-H 亚磺酰化反应。
报告了一种新的苯并咪唑基 Cu(II) 复合物在 100 °C、DMF .H2O 条件下催化咪唑并[1,2-a]吡啶与苄基/烷基/烯丙基溴化物和 CS2 的位点选择性亚磺化反应:H2O 进行硫化反应。本方法用于合成 3-亚磺酰基咪唑并[1,2-a]吡啶,产率高,底物范围广。在该方法中,通常被称为非极性小分子生物调节剂(SMB)的 CS2 被转化为有价值的亚磺酰基咪唑并[1,2-a]吡啶衍生物。此外,理论研究和实验证据还揭示了从 S,S-二苄基三硫代碳酸酯(特别是在反应过程中形成的中间体)进行位点选择性亚硫酰化的可能机理途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


