Atom swap in triple bonds via nitrogen-deletion coupling with gem-diborylalkanes
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Alkynes have played pivotal roles in numerous synthetic transformations and materials science. Here, by developing nitrogen-deletion coupling, we describe a modular synthesis of alkynes from widely accessible nitriles by swapping the N atom to a C atom in cyano groups, where lithiated gem-diborylalkanes and tert-butyl nitrite are applied sequentially. NMR analysis and crystal structure show the nature of an intermediary α-boryl lithium enamine. A diverse range of nitriles are converted into various internal and terminal alkynes within a short reaction time, including alkynes bearing bulky secondary and tertiary alkyl substituents on both sides.
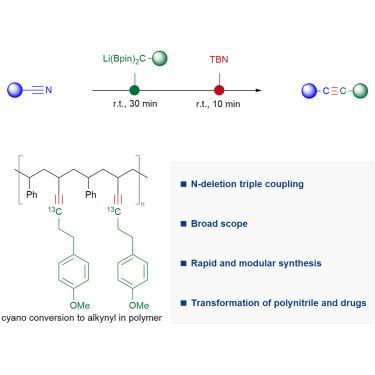
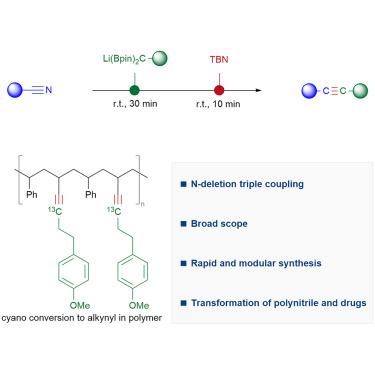
通过二硼烷的缺氮耦合实现三键中的原子交换
炔烃在众多合成转化和材料科学中发挥着关键作用。在这里,通过开发缺氮偶联,我们描述了一种通过将氰基中的 N 原子换成 C 原子,从广泛可得的腈中模块化合成炔烃的方法。核磁共振分析和晶体结构显示了中间体 α-硼烷基烯胺锂的性质。在很短的反应时间内,各种腈类都能转化为各种内部和末端炔烃,包括两侧都带有笨重的仲烷基和叔烷基取代基的炔烃。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


