A skeletally diverse library of bioactive natural-product-like compounds enabled by late-stage P450-catalyzed oxyfunctionalization
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Natural products have historically represented a major source of therapeutics and small-molecule probes for interrogating biological systems. Here, we describe the design and implementation of P450-mediated chemoenzymatic diversity-oriented synthesis (CeDOS), a strategy in which selective, regiodivergent P450-catalyzed oxyfunctionalizations are leveraged as key steps for enabling the skeletal rearrangement and diversification of a parent compound. Using this strategy and plant-derived parthenolide as the parent molecule, a structurally diverse library of over 50 unprecedented natural-product-like scaffolds was generated via divergent chemoenzymatic routes. Importantly, several members of this CeDOS library were found to exhibit notable cytotoxicity against human cancer cells as well as diversified anticancer activity profiles. This work demonstrates the power of CeDOS as a strategy for directing the construction and discovery of novel bioactive molecules, and it offers a blueprint for the broader application of this approach toward the creation and exploration of natural-product-like chemical libraries.
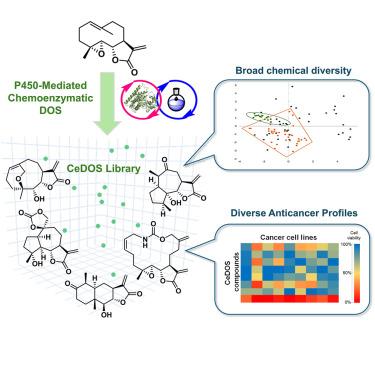
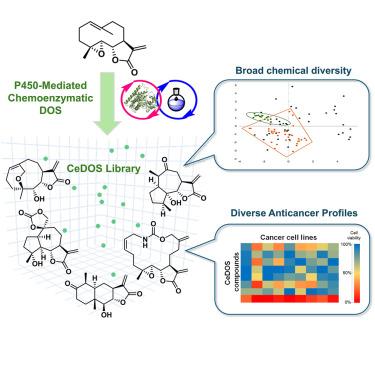
通过后期 P450 催化氧官能化作用,建立一个骨架多样化的生物活性天然产物类化合物库
天然产物历来是研究生物系统的治疗药物和小分子探针的主要来源。在这里,我们介绍了 P450 介导的化学酶多样性导向合成(CeDOS)的设计和实施,在这种策略中,选择性、区域变异性 P450 催化的氧官能化被用作实现母体化合物骨架重排和多样性的关键步骤。利用这一策略,并以植物提取的马钱子内酯为母体分子,通过不同的化学酶途径生成了一个由 50 多种前所未有的天然产品类支架组成的结构多样的化合物库。重要的是,该 CeDOS 库中的几个成员对人类癌细胞表现出显著的细胞毒性和多样化的抗癌活性。这项工作证明了 CeDOS 作为一种指导构建和发现新型生物活性分子的策略的威力,并为更广泛地应用这种方法来创建和探索类似天然产物的化学库提供了蓝图。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


