Integrin signaling in pluripotent cells acts as a gatekeeper of mouse germline entry
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Primordial germ cells (PGCs) are the precursors of gametes and the sole mechanism by which animals transmit genetic information across generations. In the mouse embryo, the transcriptional and epigenetic regulation of PGC specification has been extensively characterized. However, the initial event that triggers the soma-germline segregation remains poorly understood. Here, we uncover a critical role for the basement membrane in regulating germline entry. We show that PGCs arise in a region of the mouse embryo that lacks contact with the basement membrane, and the addition of exogenous extracellular matrix (ECM) inhibits both PGC and PGC-like cell (PGCLC) specification in mouse embryos and stem cell models, respectively. Mechanistically, we demonstrate that the engagement of β1 integrin with laminin blocks PGCLC specification by preventing the Wnt signaling–dependent down-regulation of the PGC transcriptional repressor, Otx2. In this way, the physical segregation of cells away from the basement membrane acts as a morphogenetic fate switch that controls the soma-germline bifurcation.
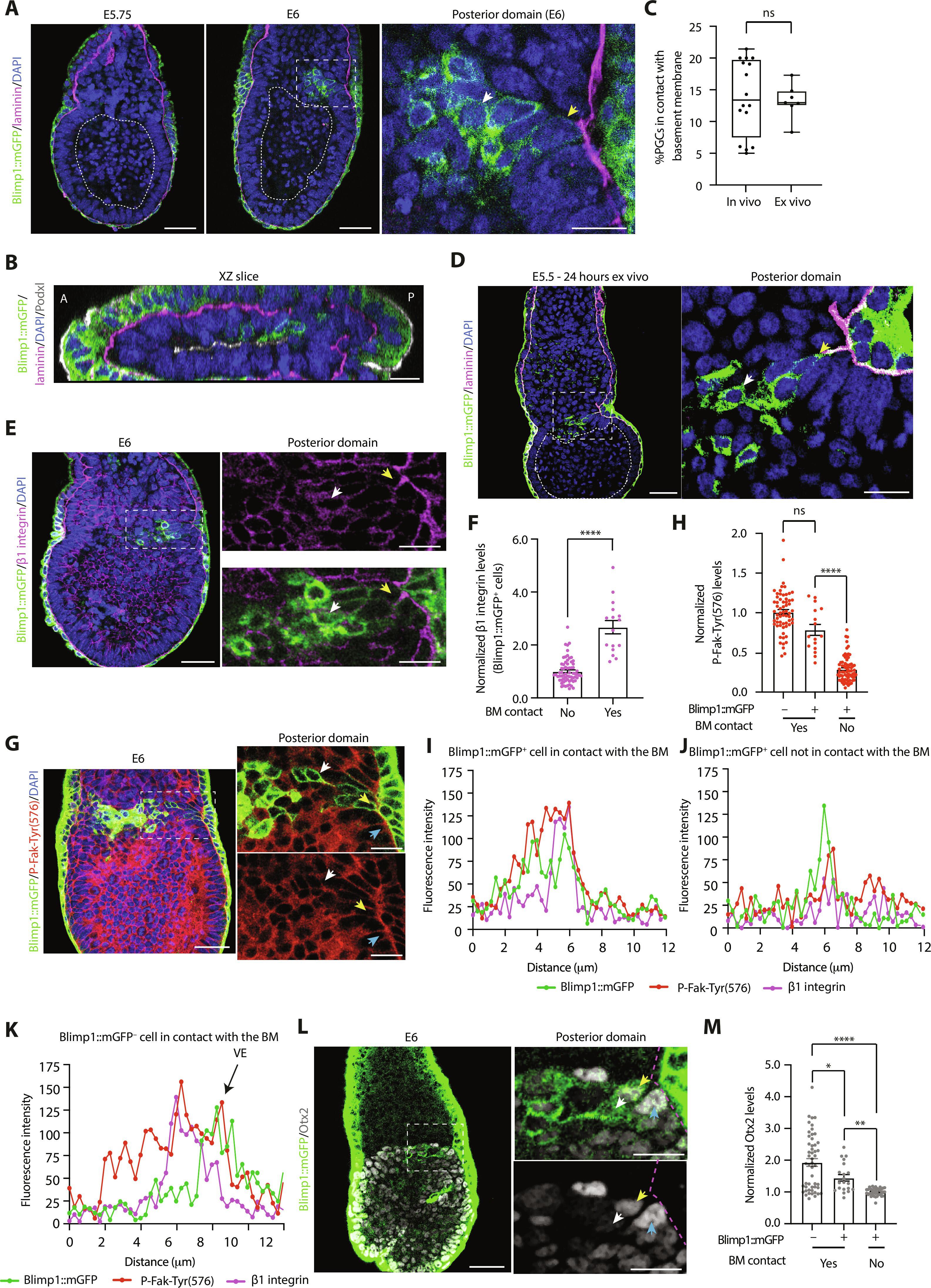
多能细胞中的整合素信号是小鼠种系进入的看门人。
原始生殖细胞(PGC)是配子的前体,也是动物跨代传递遗传信息的唯一机制。在小鼠胚胎中,PGC 分化的转录和表观遗传调控已被广泛描述。然而,人们对引发体细胞-胚系分离的初始事件仍然知之甚少。在这里,我们发现了基底膜在调节胚芽进入中的关键作用。我们发现,PGCs产生于小鼠胚胎中缺乏与基底膜接触的区域,而在小鼠胚胎和干细胞模型中,添加外源细胞外基质(ECM)会分别抑制PGC和PGC样细胞(PGCLC)的分化。从机理上讲,我们证明了β1整合素与层粘连蛋白的接合是通过阻止Wnt信号依赖的PGC转录抑制因子Otx2的下调来阻止PGCLC的分化。这样,远离基底膜的细胞物理分离就成了一种形态发生命运开关,控制着骨干-胚根分叉。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


