Discovery of CO2 tolerance genes associated with virulence in the fungal pathogen Cryptococcus neoformans
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
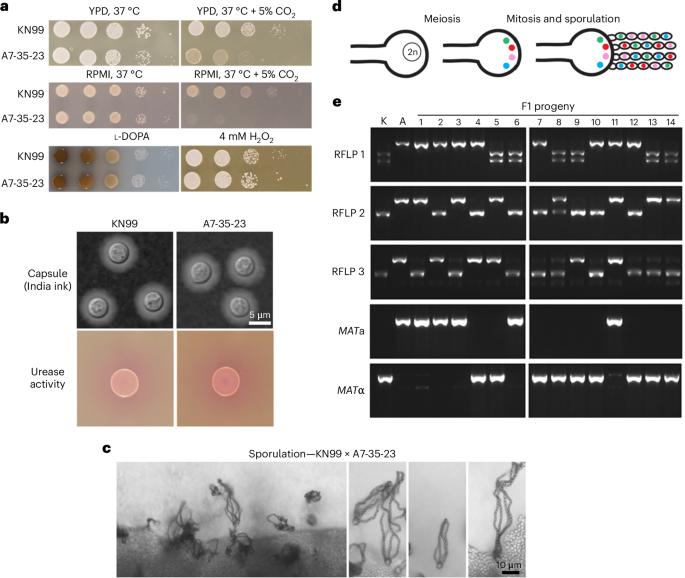
Cryptococcus neoformans is a ubiquitous soil fungus and airborne pathogen that causes over 180,000 deaths each year. Cryptococcus must adapt to host CO2 levels to cause disease, but the genetic basis for this adaptation is unknown. We utilized quantitative trait loci mapping with 374 progeny from a cross between a CO2-tolerant clinical isolate and a CO2-sensitive environmental isolate to identify genetic regions regulating CO2 tolerance. To identify specific quantitative trait genes, we applied fine mapping through bulk segregant analysis of near-isogenic progeny with distinct tolerance levels to CO2. We found that virulence among near-isogenic strains in a murine model of cryptococcosis correlated with CO2 tolerance. Moreover, we discovered that sensitive strains may adapt in vivo to become more CO2 tolerant and more virulent. These findings highlight the underappreciated role of CO2 tolerance and its importance in the ability of an opportunistic environmental pathogen to cause disease. Quantitative trait loci mapping reveals that tolerance to host CO2 is critical for virulence of the human fungal pathogen Cryptococcus neoformans.
发现与真菌病原体新生隐球菌毒力有关的二氧化碳耐受基因
新型隐球菌是一种无处不在的土壤真菌和空气传播病原体,每年导致超过 18 万人死亡。隐球菌必须适应寄主的二氧化碳水平才能致病,但这种适应的遗传基础尚不清楚。我们利用对二氧化碳耐受的临床分离株和对二氧化碳敏感的环境分离株杂交产生的 374 个后代的数量性状位点图谱来确定调控二氧化碳耐受性的遗传区域。为了确定特定的数量性状基因,我们对具有不同二氧化碳耐受水平的近等基因后代进行了大量分离分析,从而进行了精细图谱绘制。我们发现,在小鼠隐球菌病模型中,近等基因株的毒力与二氧化碳耐受性相关。此外,我们还发现,敏感菌株可能会在体内发生适应性变化,变得更耐受二氧化碳,毒性也更强。这些发现凸显了二氧化碳耐受性在机会性环境病原体致病能力中被低估的作用及其重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


