Randomized, controlled, multi-center phase II study of postoperative enoxaparin treatment for venous thromboembolism prophylaxis in patients undergoing surgery for hepatobiliary-pancreatic malignancies
Abstract
Purpose
Postoperative venous thromboembolism (VTE) risk is pronounced after abdominal cancer surgery. Enoxaparin shows promise in preventing VTE in gastrointestinal, gynecological, and urological cancers, but its application after surgery for hepatobiliary-pancreatic malignancy has been under-evaluated due to bleeding concerns. We confirmed the safety of enoxaparin administration in patients undergoing curative hepatobiliary-pancreatic surgery for malignancies in a prospective, multi-center, phase I study.
Methods
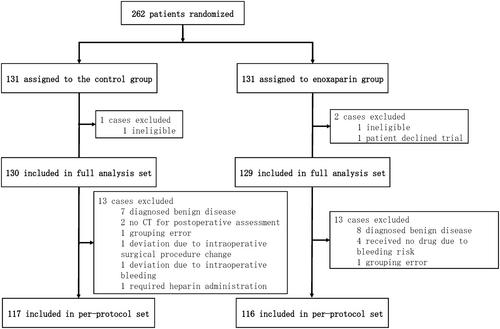
The study was conducted from April 2015 to May 2021 across eight specialized centers. Patients (n = 262) were randomized to enoxaparin prophylaxis given postoperatively for 8 days (n = 131) or control (n = 131). The primary endpoint was the efficacy in reducing VTE. Secondary endpoints examined safety.
Results
The full analysis set included 259 patients (131 control, 129 enoxaparin). The per-protocol population included 233 patients (117 control, 116 enoxaparin). Most cases were hepatic malignancies (111 control, 111 enoxaparin). The median administration duration of enoxaparin was 7 days, with 92% receiving 4000 units/day. Despite a reduction in the relative risk (RR) of VTE due to postoperative enoxaparin administration, the results were not significant (control: four cases, 3.4% vs. treatment: two cases, 1.7%; RR 0.50, 95% CI 0.09–2.70; p = 0.6834). No significant difference was found in the incidence of bleeding events (control: five cases, 4.3% vs. treatment: five cases, 4.3%, RR 1.00, 95% CI 0.53–1.89; p = 1.0000).
Conclusions
The perioperative administration of enoxaparin in hepatobiliary-pancreatic malignancies is feasible and safe. However, further case accumulation and investigation are necessary to assess its potential in reducing the occurrence of VTE.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


