Origins of enhanced oxygen reduction activity of transition metal nitrides
IF 37.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
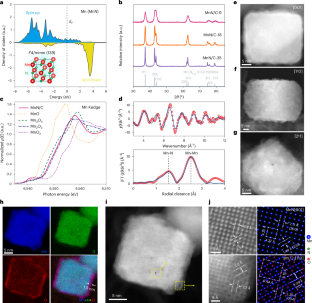
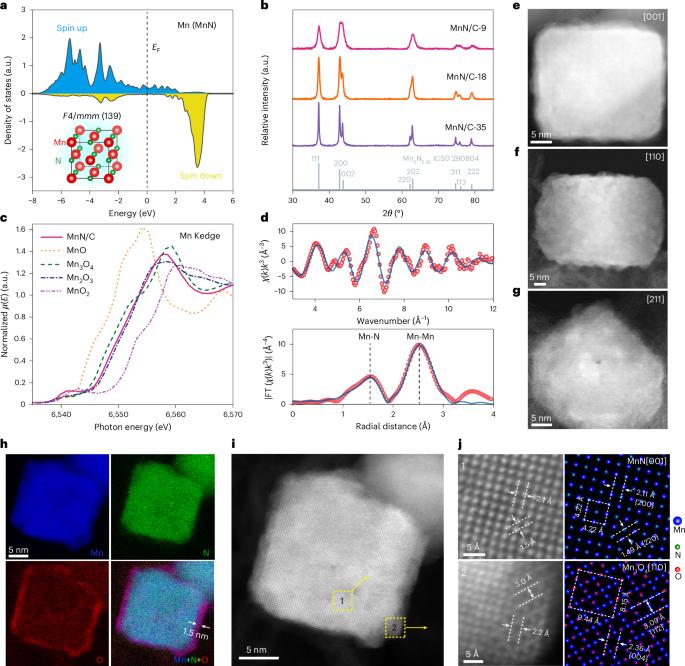
Transition metal nitride (TMN-) based materials have recently emerged as promising non-precious-metal-containing electrocatalysts for the oxygen reduction reaction (ORR) in alkaline media. However, the lack of fundamental understanding of the oxide surface has limited insights into structure–(re)activity relationships and rational catalyst design. Here we demonstrate how a well-defined TMN can dictate/control the as-formed oxide surface and the resulting ORR electrocatalytic activity. Structural characterization of MnN nanocuboids revealed that an electrocatalytically active Mn3O4 shell grew epitaxially on the MnN core, with an expansive strain along the [010] direction to the surface Mn3O4. The strained Mn3O4 shell on the MnN core exhibited an intrinsic activity that was over 300% higher than that of pure Mn3O4. A combined electrochemical and computational investigation indicated/suggested that the enhancement probably originates from a more hydroxylated oxide surface resulting from the expansive strain. This work establishes a clear and definitive atomistic picture of the nitride/oxide interface and provides a comprehensive mechanistic understanding of the structure–reactivity relationship in TMNs, critical for other catalytic interfaces for different electrochemical processes. While transition metal nitrides are promising low-cost electrocatalysts for the oxygen reduction reaction in alkaline media, a fundamental understanding of their activity is still lacking. Here MnN nanocuboids with well-defined surface structures are investigated, providing atomistic insight and mechanistic understanding.
过渡金属氮化物氧还原活性增强的起源
基于过渡金属氮化物(TMN-)的材料最近已成为在碱性介质中进行氧还原反应(ORR)的前景看好的非贵金属电催化剂。然而,由于缺乏对氧化物表面的基本了解,限制了对结构-(再)活性关系和催化剂合理设计的深入了解。在此,我们展示了定义明确的 TMN 如何决定/控制所形成的氧化物表面以及由此产生的 ORR 电催化活性。MnN 纳米立方体的结构表征显示,具有电催化活性的 Mn3O4 外壳在 MnN 内核上外延生长,并沿 [010] 方向与表面 Mn3O4 形成扩张应变。MnN 内核上的应变 Mn3O4 壳显示出比纯 Mn3O4 高出 300% 以上的内在活性。电化学和计算研究的综合结果表明/暗示,活性的提高可能源于膨胀应变使氧化物表面羟基化程度更高。这项研究为氮化物/氧化物界面建立了清晰明确的原子论图景,并为 TMNs 的结构-活性关系提供了全面的机理认识,这对于不同电化学过程中的其他催化界面至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


