Mucosal adenovirus vaccine boosting elicits IgA and durably prevents XBB.1.16 infection in nonhuman primates
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
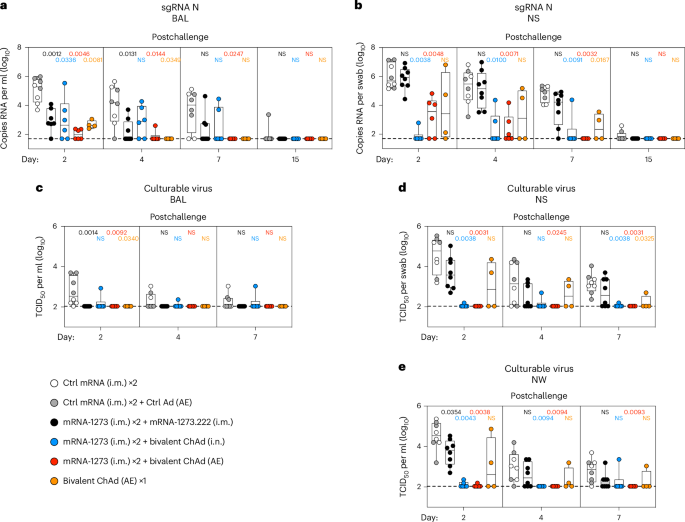
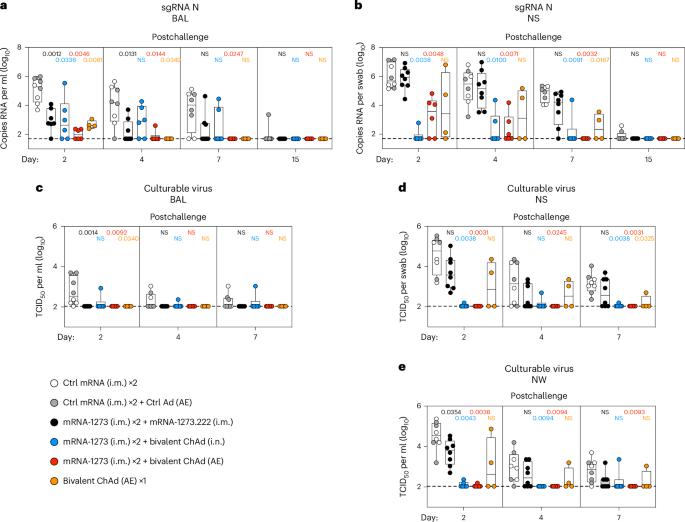
A mucosal route of vaccination could prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication at the site of infection and limit transmission. We compared protection against heterologous XBB.1.16 challenge in nonhuman primates (NHPs) ~5 months following intramuscular boosting with bivalent mRNA encoding WA1 and BA.5 spike proteins or mucosal boosting with a WA1–BA.5 bivalent chimpanzee adenoviral-vectored vaccine delivered by intranasal or aerosol device. NHPs boosted by either mucosal route had minimal virus replication in the nose and lungs, respectively. By contrast, protection by intramuscular mRNA was limited to the lower airways. The mucosally delivered vaccine elicited durable airway IgG and IgA responses and, unlike the intramuscular mRNA vaccine, induced spike-specific B cells in the lungs. IgG, IgA and T cell responses correlated with protection in the lungs, whereas mucosal IgA alone correlated with upper airway protection. This study highlights differential mucosal and serum correlates of protection and how mucosal vaccines can durably prevent infection against SARS-CoV-2. Seder and colleagues show that mucosal adenoviral-vectored vaccine boosting durably prevents infection in nonhuman primates of the highly transmissible, heterologous XBB.1.16 strain of SARS-CoV-2.
在非人灵长类动物中,粘膜腺病毒疫苗强化可激发 IgA 并持久预防 XBB.1.16 感染
通过粘膜途径接种疫苗可以防止严重急性呼吸系统综合征冠状病毒 2(SARS-CoV-2)在感染部位复制并限制传播。我们比较了非人灵长类动物(NHPs)在肌肉注射编码WA1和BA.5尖峰蛋白的二价mRNA或通过鼻内或气雾装置接种WA1-BA.5二价黑猩猩腺病毒载体疫苗约5个月后对异源XBB.1.16挑战的保护。通过这两种粘膜途径接种疫苗的黑猩猩分别在鼻腔和肺部复制了极少量的病毒。相比之下,肌肉注射 mRNA 的保护作用仅限于下呼吸道。粘膜给药疫苗可引起持久的气道 IgG 和 IgA 反应,与肌肉注射 mRNA 疫苗不同的是,粘膜给药疫苗可诱导肺部的尖峰特异性 B 细胞。IgG、IgA和T细胞反应与肺部保护相关,而仅粘膜IgA与上呼吸道保护相关。这项研究强调了不同的粘膜和血清保护相关性,以及粘膜疫苗如何能持久预防 SARS-CoV-2 感染。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


