Pancreatic islet cells in microfluidic-spun hydrogel microfibers for the treatment of diabetes
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Islet transplantation has been developed as an effective cell therapy strategy to treat the progressive life-threatening disease Type 1 diabetes (T1DM). To mimic the natural islets and achieve immune isolation, hydrogel encapsulation of multiple islet cell types is the current endeavor. Here, we present a microfiber loading with pancreatic α and β cells by microfluidic spinning for diabetes treatment. Benefiting from microfluidic technology, the cells could be controllably and continuously loaded in the alginate and methacrylated hyaluronic acid (Alg-HAMA) microfiber and maintained their high bioactivity. The resultant microfiber could then hold the capacity of dual-mode glucose responsiveness attributed to the glucagon and insulin secreted by the encapsulated pancreatic α and β cells. After transplantation into the brown adipose tissue (BAT), these cell-laden microfibers showed successful blood glucose control in rodents and avoided the occurrence of hypoglycemia. These results conceived that the multicellular microfibers are expected to provide new insight into artificial islet preparation, diabetes treatment, and regenerative medicine as well as tissue engineering.
Statement of significance
- •The microfibers were generated with a double network of alginate and methacrylated hyaluronic acid.
- •The microfibers could simultaneously encapsulate pancreatic α and β cells and showed dual-mode glucose responsiveness.
- •The cell-laden microfibers maintained a long-term hormone-releasing function in vivo.
- •Cell-laden microfibers transplanted into diabetic mice could achieve glycemic control for 6 weeks.
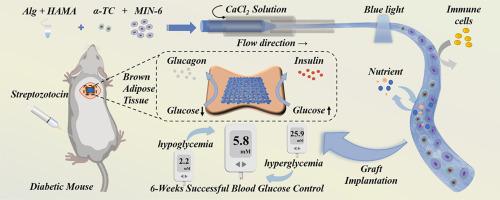
用于治疗糖尿病的微流体纺丝水凝胶微纤维中的胰岛细胞。
胰岛移植已被开发为一种有效的细胞治疗策略,用于治疗进展性危及生命的 1 型糖尿病(T1DM)。为了模拟天然胰岛并实现免疫隔离,目前正在努力实现多种胰岛细胞类型的水凝胶封装。在此,我们介绍了一种通过微流控纺丝技术装载胰腺α和β细胞的微纤维,用于糖尿病治疗。得益于微流体技术,细胞可以可控地、持续地负载在海藻酸盐和甲基丙烯酸化透明质酸(Alg-HAMA)微纤维中,并保持其较高的生物活性。由此产生的微纤维可保持双模式葡萄糖反应能力,这归功于被包裹的胰腺α和β细胞分泌的胰高血糖素和胰岛素。在移植到棕色脂肪组织(BAT)后,这些含有细胞的微纤维成功地控制了啮齿动物的血糖,避免了低血糖的发生。这些结果预示着多细胞微纤维有望为人工胰岛制备、糖尿病治疗、再生医学和组织工程提供新的见解。意义说明:该微纤维由海藻酸和甲基丙烯酸化透明质酸双重网络生成。微纤维可同时包裹胰腺α和β细胞,并显示出双模式葡萄糖响应性。含有细胞的微纤维在体内可长期保持激素释放功能。将含有细胞的微纤维移植到糖尿病小鼠体内,可在6周内控制血糖。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


