Telomere stabilization by metformin mitigates the progression of atherosclerosis via the AMPK-dependent p-PGC-1α pathway
IF 12.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Telomere dysfunction is a well-known molecular trigger of senescence and has been associated with various age-related diseases, including atherosclerosis. However, the mechanisms involved have not yet been elucidated, and the extent to which telomeres contribute to atherosclerosis is unknown. Therefore, we investigated the mechanism of metformin-induced telomere stabilization and the ability of metformin to inhibit vascular smooth muscle cell (VSMC) senescence caused by advanced atherosclerosis. The present study revealed that metformin inhibited the phenotypes of atherosclerosis and senescence in VSMCs. Metformin increased the phosphorylation of AMPK-dependent PGC-1α and thus increased telomerase activity and the protein level of TERT in OA-treated VSMCs. Mechanistically, the phosphorylation of AMPK and PGC-1α by metformin not only enhanced telomere function but also increased the protein level of TERT, whereas TERT knockdown accelerated the development of atherosclerosis and senescent phenotypes in OA-treated VSMCs regardless of metformin treatment. Furthermore, the in vivo results showed that metformin attenuated the formation of atherosclerotic plaque markers in the aortas of HFD-fed ApoE KO mice. Although metformin did not reduce plaque size, it inhibited the phosphorylation of the AMPK/PGC-1α/TERT signaling cascade, which is associated with the maintenance and progression of plaque formation, in HFD-fed ApoE KO mice. Accordingly, metformin inhibited atherosclerosis-associated phenotypes in vitro and in vivo. These observations show that the enhancement of telomere function by metformin is involved in specific signaling pathways during the progression of atherosclerosis. These findings suggest that telomere stabilization by metformin via the AMPK/p-PGC-1α pathway might provide a strategy for developing therapeutics against vascular diseases such as atherosclerosis. Atherosclerosis is a condition where fats build up in arteries, causing heart disease. A study investigates the effect of Metformin, a diabetes drug, on this condition. Researchers studied how Metformin affects the aging of vascular smooth muscle cells. The study used cell cultures and mice to examine Metformin’s effect on cell aging and atherosclerosis. The experiment involved treating cells and mice with Metformin and observing changes in inflammation, plaque formation, and cell aging. The findings showed that Metformin slows down aging in VSMCs and reduces plaque formation in mice, indicating it might be useful beyond diabetes treatment. The study concludes that Metformin’s ability to enhance cell health and lessen atherosclerosis could be due to its impact on cell aging processes. This finding paves the way for using Metformin to prevent heart disease. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
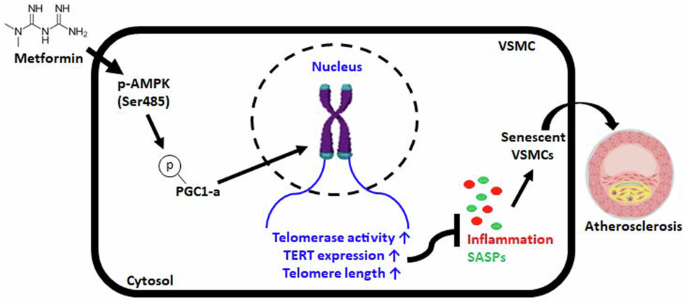
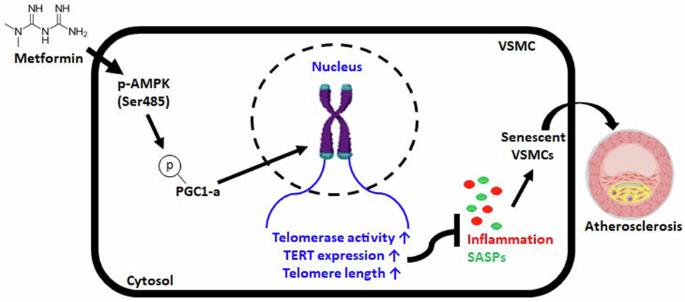
二甲双胍通过 AMPK 依赖性 p-PGC-1α 通路稳定端粒,从而缓解动脉粥样硬化的进展。
端粒功能障碍是众所周知的衰老分子诱因,与包括动脉粥样硬化在内的各种老年相关疾病有关。然而,其中的机制尚未阐明,端粒对动脉粥样硬化的影响程度也不得而知。因此,我们研究了二甲双胍诱导端粒稳定的机制以及二甲双胍抑制晚期动脉粥样硬化引起的血管平滑肌细胞(VSMC)衰老的能力。本研究发现,二甲双胍抑制了动脉粥样硬化和血管平滑肌细胞衰老的表型。二甲双胍增加了依赖于AMPK的PGC-1α的磷酸化,从而提高了端粒酶活性和OA处理的VSMC中TERT的蛋白水平。从机理上讲,二甲双胍对AMPK和PGC-1α的磷酸化不仅增强了端粒功能,还提高了TERT的蛋白水平,而无论二甲双胍处理与否,敲除TERT都会加速OA处理VSMC的动脉粥样硬化和衰老表型的发展。此外,体内研究结果表明,二甲双胍可减轻HFD喂养的载脂蛋白E KO小鼠主动脉中动脉粥样硬化斑块标志物的形成。虽然二甲双胍没有减少斑块的大小,但它抑制了AMPK/PGC-1α/TERT信号级联的磷酸化,而AMPK/PGC-1α/TERT信号级联与HFD喂养载脂蛋白E KO小鼠斑块形成的维持和进展有关。因此,二甲双胍抑制了体外和体内与动脉粥样硬化相关的表型。这些观察结果表明,二甲双胍对端粒功能的增强参与了动脉粥样硬化进展过程中的特定信号通路。这些研究结果表明,二甲双胍通过AMPK/p-PGC-1α途径稳定端粒可能为开发针对动脉粥样硬化等血管疾病的疗法提供了一种策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


