Individual bacterial cells can use spatial sensing of chemical gradients to direct chemotaxis on surfaces
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Swimming bacteria navigate chemical gradients using temporal sensing to detect changes in concentration over time. Here we show that surface-attached bacteria use a fundamentally different mode of sensing during chemotaxis. We combined microfluidic experiments, massively parallel cell tracking and fluorescent reporters to study how Pseudomonas aeruginosa senses chemical gradients during pili-based ‘twitching’ chemotaxis on surfaces. Unlike swimming cells, we found that temporal changes in concentration did not induce motility changes in twitching cells. We then quantified the chemotactic behaviour of stationary cells by following changes in the sub-cellular localization of fluorescent proteins as cells are exposed to a gradient that alternates direction. These experiments revealed that P. aeruginosa cells can directly sense differences in concentration across the lengths of their bodies, even in the presence of strong temporal fluctuations. Our work thus overturns the widely held notion that bacterial cells are too small to directly sense chemical gradients in space. Microfluidic experiments reveal that surface-attached Pseudomonas aeruginosa cells directly sense differences in chemical concentration across the length of their cell bodies to guide pili-based chemotaxis.
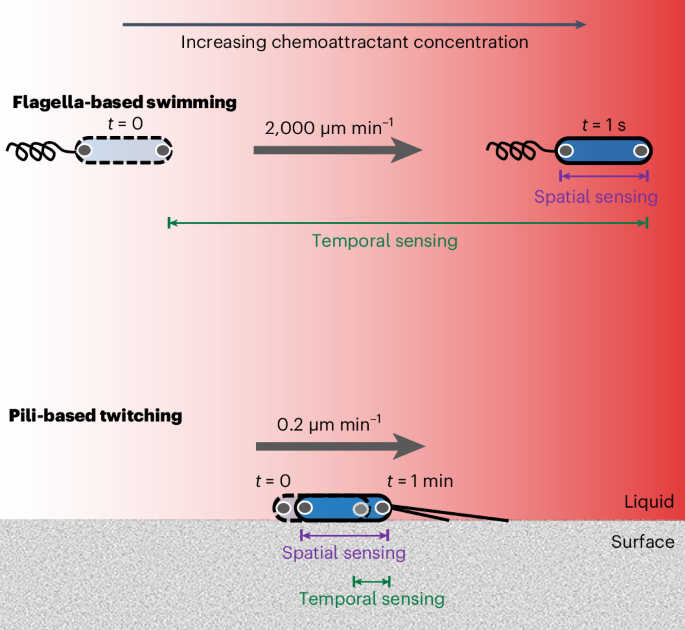
单个细菌细胞可以利用对化学梯度的空间感知来引导表面的趋化运动
游动细菌利用时间感应来探测化学梯度随时间的浓度变化。在这里,我们展示了表面附着细菌在趋化过程中使用的一种根本不同的感知模式。我们将微流体实验、大规模并行细胞追踪和荧光报告结合起来,研究了铜绿假单胞菌在基于绒毛的表面 "抽动 "趋化过程中如何感知化学梯度。与游动细胞不同,我们发现浓度的时间变化不会引起抽动细胞的运动变化。然后,我们通过跟踪细胞暴露在方向交替的梯度中时荧光蛋白亚细胞定位的变化,量化了静止细胞的趋化行为。这些实验揭示了铜绿微囊藻细胞即使在强烈的时间波动下,也能直接感知整个细胞长度上的浓度差异。因此,我们的研究推翻了人们普遍认为的细菌细胞太小而无法直接感知空间化学梯度的观点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


