In situ p-block protective layer plating in carbonate-based electrolytes enables stable cell cycling in anode-free lithium batteries
IF 37.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
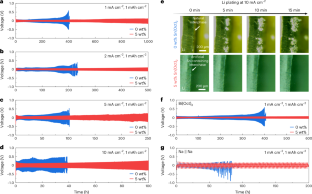
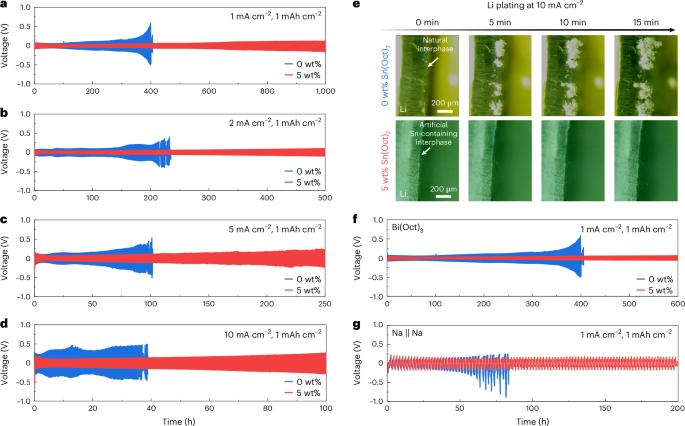
‘Anode-free’ Li metal batteries offer the highest possible energy density but face low Li coulombic efficiency when operated in carbonate electrolytes. Here we report a performance improvement of anode-free Li metal batteries using p-block tin octoate additive in the carbonate electrolyte. We show that the preferential adsorption of the octoate moiety on the Cu substrate induces the construction of a carbonate-less protective layer, which inhibits the side reactions and contributes to the uniform Li plating. In the mean time, the reduction of Sn2+ at the initial charging process builds a stable lithophilic layer of Cu6Sn5 alloy and Sn, improving the affinity between the Li and the Cu substrate. Notably, anode-free Li metal pouch cells with tin octoate additive demonstrate good cycling stability with a high coulombic efficiency of ~99.1%. Furthermore, this in situ p-block layer plating strategy is also demonstrated with other types of p-block metal octoate, as well as a Na metal battery system, demonstrating the high level of universality. A p-block metal octoate additive in carbonate electrolytes enables the reversible plating/stripping of alkali metal in anode-free batteries by forming a protective layer with a preferentially adsorbed octoate moiety and uniformly plated p-block metal.
在碳酸盐基电解质中原位电镀 p 块保护层可实现无阳极锂电池的稳定电池循环
无阳极 "金属锂电池具有最高的能量密度,但在碳酸盐电解质中运行时,锂的库仑效率较低。在此,我们报告了在碳酸盐电解质中使用辛酸锡添加剂提高无阳极金属锂电池性能的方法。我们的研究表明,辛酸锡分子在铜基板上的优先吸附作用诱导构建了无碳酸盐保护层,从而抑制了副反应并促进了锂的均匀电镀。与此同时,初始充电过程中 Sn2+ 的还原作用在 Cu6Sn5 合金和 Sn 之间形成了稳定的嗜石层,从而提高了锂与 Cu 基底之间的亲和力。值得注意的是,使用辛酸锡添加剂的无阳极锂金属袋电池表现出良好的循环稳定性,库仑效率高达约 99.1%。此外,这种原位 p 块层电镀策略还在其他类型的 p 块八酸盐金属以及 Na 金属电池系统中得到了验证,证明了其高度的通用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


