Revealing the degradation pathways of layered Li-rich oxide cathodes
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
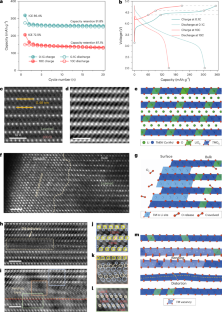
Layered lithium-rich transition metal oxides are promising cathode candidates for high-energy-density lithium batteries due to the redox contributions from transition metal cations and oxygen anions. However, their practical application is hindered by gradual capacity fading and voltage decay. Although oxygen loss and phase transformation are recognized as primary factors, the structural deterioration, chemical rearrangement, kinetic and thermodynamic effects remain unclear. Here we integrate analysis of morphological, structural and oxidation state evolution from individual atoms to secondary particles. By performing nanoscale to microscale characterizations, distinct structural change pathways associated with intraparticle heterogeneous reactions are identified. The high level of oxygen defects formed throughout the particle by slow electrochemical activation triggers progressive phase transformation and the formation of nanovoids. Ultrafast lithium (de)intercalation leads to oxygen-distortion-dominated lattice displacement, transition metal ion dissolution and lithium site variation. These inhomogeneous and irreversible structural changes are responsible for the low initial Coulombic efficiency, and ongoing particle cracking and expansion in the subsequent cycles. This work employs nano- to microscale characterization to identify different structural change pathways associated with non-homogeneous reactions within the particles, and explores differences in the failure mechanisms of lithium-rich transition metal oxide materials at different current densities.

揭示层状富锂氧化物阴极的降解途径
由于过渡金属阳离子和氧阴离子的氧化还原作用,层状富锂过渡金属氧化物有望成为高能量密度锂电池的正极候选材料。然而,它们的实际应用却受到容量逐渐衰减和电压衰减的阻碍。虽然氧损耗和相变被认为是主要因素,但结构退化、化学重排、动力学和热力学效应仍不清楚。在这里,我们综合分析了从单个原子到次级粒子的形态、结构和氧化态演变。通过从纳米尺度到微观尺度的表征,我们确定了与颗粒内异质反应相关的独特结构变化途径。通过缓慢的电化学活化在整个颗粒中形成的高水平氧缺陷引发了渐进的相变并形成了纳米固体。超快的锂(脱)插层导致以氧畸变为主的晶格位移、过渡金属离子溶解和锂位点变化。这些不均匀和不可逆的结构变化是造成初始库仑效率低以及在随后的循环中颗粒不断开裂和膨胀的原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


