Plasticity variable collagen-PEG interpenetrating networks modulate cell spreading
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
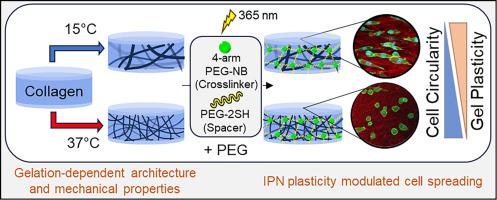
The extracellular matrix protein collagen I has been used extensively in the field of biomaterials due to its inherent biocompatibility and unique viscoelastic and mechanical properties. Collagen I self-assembly into fibers and networks is environmentally sensitive to gelation conditions such as temperature, resulting in gels with distinct network architectures and mechanical properties. Despite this, collagen gels are not suitable for many applications given their relatively low storage modulus. We have prepared collagen-poly(ethylene glycol) [PEG] interpenetrating network (IPN) hydrogels to reinforce the collagen network, which also induces changes to network plasticity, a recent focus of study in cell-matrix interactions. Here, we prepare collagen/PEG IPNs, varying collagen concentration and collagen gelation temperature to assess changes in microarchitecture and mechanical properties of these networks. By tuning these parameters, IPNs with a range of stiffness, plasticity and pore size are obtained. Cell studies suggest that matrix plasticity is a key determinant of cell behavior, including cell elongation, on these gels. This work presents a natural/synthetic biocompatible matrix that retains the unique structural properties of collagen networks with increased storage modulus and tunable plasticity. The described IPN materials will be of use for applications in which control of cell spreading is desirable, as only minimal changes in sample preparation lead to changes in cell spreading and circularity. Additionally, this study contributes to our understanding of the connection between collagen self-assembly conditions and matrix structural and mechanical properties and presents them as useful tools for the design of other collagen based biomaterials.
Statement of significance
We developed a collagen-poly(ethylene glycol) interpenetrating network (IPN) platform that retains native collagen architecture and biocompatibility but provides higher stiffness and tunable plasticity. With minor changes in collagen gelation temperature or concentration, IPN gels with a range of plasticity, storage modulus, and pore size can be obtained. The tunable plasticity of the gels is shown to modulate cell spreading, with a greater proportion of elongated cells on the most plastic of IPNs, supporting the assertion that matrix plasticity is a key determinant of cell spreading. The material can be of use for situations where control of cell spreading is desired with minimal intervention, and the findings herein may be used to develop similar collagen based IPN platforms.
可塑性可变胶原蛋白-聚乙二醇互穿网络调节细胞扩散
细胞外基质蛋白质胶原蛋白 I 因其固有的生物相容性和独特的粘弹性及机械特性,已被广泛应用于生物材料领域。胶原蛋白 I 自组装成纤维和网络对温度等凝胶化条件的环境敏感,从而形成具有独特网络结构和机械性能的凝胶。尽管如此,由于胶原蛋白凝胶的储存模量相对较低,因此并不适用于许多应用领域。我们制备了胶原蛋白-聚乙二醇(PEG)互穿网络(IPN)水凝胶来增强胶原蛋白网络,这也诱导了网络可塑性的变化,这是细胞-基质相互作用的最新研究重点。在此,我们制备了不同胶原浓度和胶原凝胶化温度的胶原/PEG IPN,以评估这些网络的微观结构和机械性能的变化。通过调整这些参数,可获得具有不同硬度、可塑性和孔径的 IPN。细胞研究表明,基质可塑性是决定细胞在这些凝胶上的行为(包括细胞伸长)的关键因素。这项研究提出了一种天然/合成的生物相容性基质,它保留了胶原蛋白网络的独特结构特性,并增加了储存模量和可调塑性。所描述的 IPN 材料将用于需要控制细胞铺展的应用中,因为样品制备中的微小变化就会导致细胞铺展和圆度的变化。此外,这项研究还有助于我们理解胶原蛋白自组装条件与基质结构和机械性能之间的联系,并将其作为设计其他基于胶原蛋白的生物材料的有用工具。意义说明:我们开发了一种胶原蛋白-聚乙二醇互穿网络(IPN)平台,它保留了原生胶原蛋白的结构和生物相容性,但具有更高的硬度和可调可塑性。只需稍稍改变胶原蛋白的凝胶化温度或浓度,就能获得具有不同可塑性、储存模量和孔径的 IPN 凝胶。研究表明,凝胶的可调可塑性可调节细胞的扩散,在可塑性最强的 IPN 上,伸长细胞的比例更大,这支持了基质可塑性是细胞扩散的关键决定因素这一观点。这种材料可用于需要以最小干预控制细胞扩散的情况,本文的研究结果可用于开发类似的基于胶原蛋白的 IPN 平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


