The effect of novel β-lactam derivatives synthesized from substituted phenethylamines on resistance genes of MRSA isolates
IF 2.1
4区 医学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
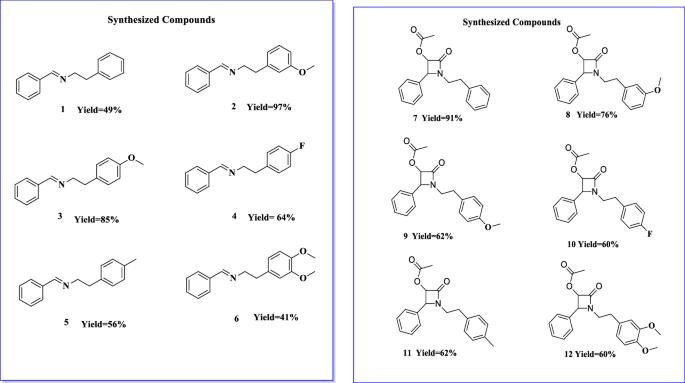
This study focuses on the activity of previously reported imine and β-lactam derivatives against methicillin-resistant Staphylococcus aureus (MRSA) isolates. The presence of mecA and blaZ genes in the isolates was determined, and the minimum inhibitory concentration (MIC) values were determined based on the antibacterial activity against these isolates. Active compounds were selected and their ability to act against resistant isolates in vitro was determined. Concurrently, biochemical (nitrocefin) and molecular (qRT-PCR) tests were used to investigate the ability of the compounds to induce resistance genes in MRSA isolates. The cytotoxicity of the compounds on human dermal fibroblasts (HDF) was investigated. The MIC values of compounds (10) and (12) against MSSA and MRSA isolates were 7.81 and 15.62 μg ml−1, respectively. The most active compounds were identified as (10) and (12), and it was observed that the isolates did not develop resistance to these compounds in vitro. These compounds were found to inhibit β-lactamase, reduce the expression of resistance genes, and exhibit reduced HDF cell toxicity in a dose-dependent manner. According to the findings of the study, it can be concluded that these compounds show promise as hits with an interesting mechanism of action for further chemical modifications to develop new MRSA inhibitors.
由取代的苯乙胺合成的新型β-内酰胺衍生物对 MRSA 分离物耐药基因的影响。
本研究的重点是以前报道过的亚胺和β-内酰胺衍生物对耐甲氧西林金黄色葡萄球菌(MRSA)分离物的活性。确定了分离物中是否存在 mecA 和 blaZ 基因,并根据对这些分离物的抗菌活性确定了最低抑菌浓度 (MIC) 值。筛选出活性化合物,并确定其体外抗耐药性分离物的能力。同时,还采用了生化(硝化纤维素)和分子(qRT-PCR)测试来研究化合物诱导 MRSA 分离物耐药基因的能力。研究了化合物对人真皮成纤维细胞(HDF)的细胞毒性。化合物(10)和(12)对 MSSA 和 MRSA 分离物的 MIC 值分别为 7.81 和 15.62 μg ml-1。经鉴定,活性最强的化合物是(10)和(12),而且据观察,分离物在体外对这些化合物没有产生抗药性。研究发现,这些化合物能抑制β-内酰胺酶,减少耐药基因的表达,并以剂量依赖的方式降低 HDF 细胞的毒性。根据这项研究的结果,可以得出结论:这些化合物有望成为具有有趣作用机制的新药,可进一步进行化学修饰,以开发新的 MRSA 抑制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Antibiotics
医学-免疫学
CiteScore
6.60
自引率
3.00%
发文量
87
审稿时长
1 months
期刊介绍:
The Journal of Antibiotics seeks to promote research on antibiotics and related types of biologically active substances and publishes Articles, Review Articles, Brief Communication, Correspondence and other specially commissioned reports. The Journal of Antibiotics accepts papers on biochemical, chemical, microbiological and pharmacological studies. However, studies regarding human therapy do not fall under the journal’s scope. Contributions regarding recently discovered antibiotics and biologically active microbial products are particularly encouraged. Topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Discovery of new antibiotics and related types of biologically active substances
Production, isolation, characterization, structural elucidation, chemical synthesis and derivatization, biological activities, mechanisms of action, and structure-activity relationships of antibiotics and related types of biologically active substances
Biosynthesis, bioconversion, taxonomy and genetic studies on producing microorganisms, as well as improvement of production of antibiotics and related types of biologically active substances
Novel physical, chemical, biochemical, microbiological or pharmacological methods for detection, assay, determination, structural elucidation and evaluation of antibiotics and related types of biologically active substances
Newly found properties, mechanisms of action and resistance-development of antibiotics and related types of biologically active substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


