Distinct binding conformations of epinephrine with α- and β-adrenergic receptors
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Agonists targeting α2-adrenergic receptors (ARs) are used to treat diverse conditions, including hypertension, attention-deficit/hyperactivity disorder, pain, panic disorders, opioid and alcohol withdrawal symptoms, and cigarette cravings. These receptors transduce signals through heterotrimeric Gi proteins. Here, we elucidated cryo-EM structures that depict α2A-AR in complex with Gi proteins, along with the endogenous agonist epinephrine or the synthetic agonist dexmedetomidine. Molecular dynamics simulations and functional studies reinforce the results of the structural revelations. Our investigation revealed that epinephrine exhibits different conformations when engaging with α-ARs and β-ARs. Furthermore, α2A-AR and β1-AR (primarily coupled to Gs, with secondary associations to Gi) were compared and found to exhibit different interactions with Gi proteins. Notably, the stability of the epinephrine–α2A-AR–Gi complex is greater than that of the dexmedetomidine–α2A-AR–Gi complex. These findings substantiate and improve our knowledge on the intricate signaling mechanisms orchestrated by ARs and concurrently shed light on the regulation of α-ARs and β-ARs by epinephrine. Our bodies have a system, the sympathetic nervous system, that uses certain chemicals to control heart rate, blood pressure, etc. These chemicals, epinephrine and norepinephrine, work by activating proteins known as adrenergic receptors. Understanding these receptors could help treat diseases like high blood pressure and ADHD. This study used a method called cryo-electron microscopy to see how epinephrine interacts with these receptors. It compared how epinephrine and a similar drug, dexmedetomidine, interact with the receptors. The study found that epinephrine binds to the α and β types of the receptors differently, which could explain their different effects. This helps us understand how drugs that mimic or block epinephrine can treat diseases. This could lead to new, more effective drugs. Future research may use these findings to design better treatments for heart diseases and other conditions. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
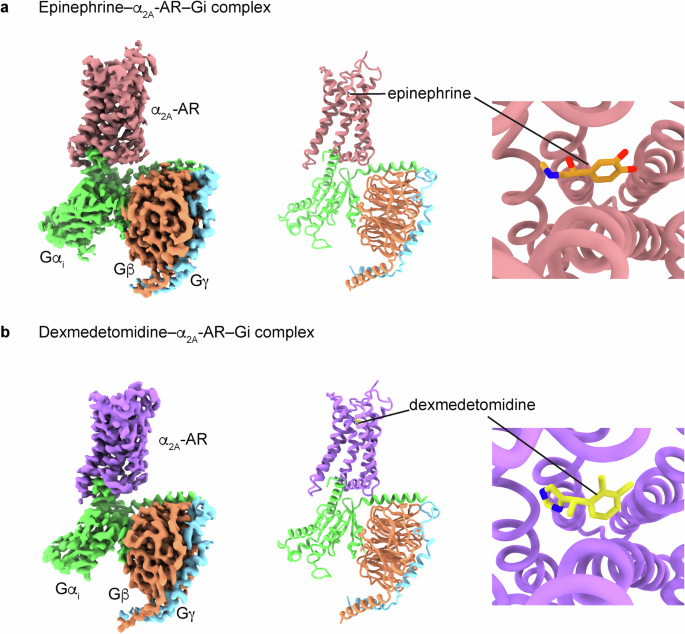
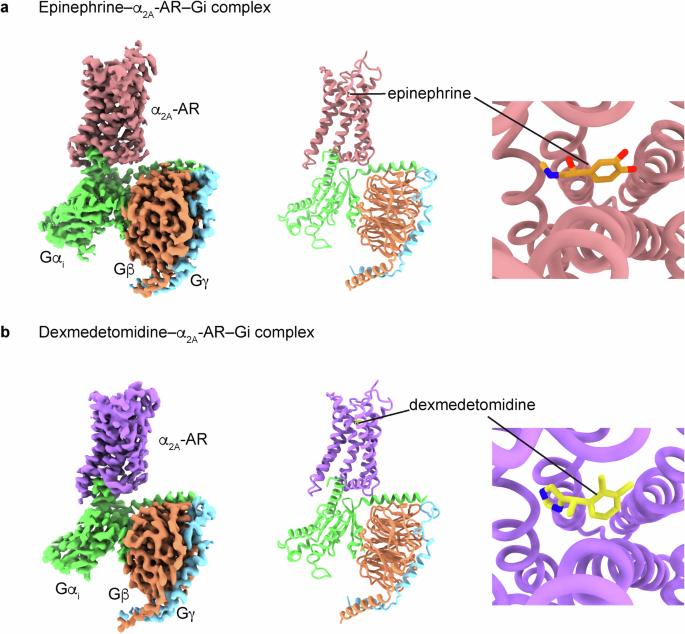
肾上腺素与 α 和 β 肾上腺素能受体的不同结合构象。
以α2肾上腺素能受体(ARs)为靶点的激动剂可用于治疗多种疾病,包括高血压、注意力缺陷/多动障碍、疼痛、恐慌症、阿片类药物和酒精戒断症状以及烟瘾。这些受体通过异三聚 Gi 蛋白传递信号。在这里,我们阐明了描述α2A-AR与Gi蛋白以及内源性激动剂肾上腺素或合成激动剂右美托咪定复合物的低温电子显微镜结构。分子动力学模拟和功能研究加强了结构揭示的结果。我们的研究发现,肾上腺素在与α-ARs 和 β-ARs 结合时呈现出不同的构象。此外,我们还比较了α2A-AR 和 β1-AR(主要与 Gs 耦合,次要与 Gi 关联),发现它们与 Gi 蛋白的相互作用各不相同。值得注意的是,肾上腺素-α2A-AR-Gi 复合物的稳定性高于右美托咪定-α2A-AR-Gi 复合物。这些发现证实并提高了我们对 ARs 错综复杂的信号转导机制的认识,同时也揭示了肾上腺素对 α-ARs 和 β-ARs 的调控作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


