Blockade of STING activation alleviates microglial dysfunction and a broad spectrum of Alzheimer’s disease pathologies
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Abnormal glial activation promotes neurodegeneration in Alzheimer’s disease (AD), the most common cause of dementia. Stimulation of the cGAS-STING pathway induces microglial dysfunction and sterile inflammation, which exacerbates AD. We showed that inhibiting STING activation can control microglia and ameliorate a wide spectrum of AD symptoms. The cGAS-STING pathway is required for the detection of ectopic DNA and the subsequent immune response. Amyloid-β (Aβ) and tau induce mitochondrial stress, which causes DNA to be released into the cytoplasm of microglia. cGAS and STING are highly expressed in Aβ plaque-associated microglia, and neuronal STING is upregulated in the brains of AD model animals. The presence of the APOE ε4 allele, an AD risk factor, also upregulated both proteins. STING activation was necessary for microglial NLRP3 activation, proinflammatory responses, and type-I-interferon responses. Pharmacological STING inhibition reduced a wide range of AD pathogenic features in AppNL-G-F/hTau double-knock-in mice. An unanticipated transcriptome shift in microglia reduced gliosis and cerebral inflammation. Significant reductions in the Aβ load, tau phosphorylation, and microglial synapse engulfment prevented memory loss. To summarize, our study describes the pathogenic mechanism of STING activation as well as its potential as a therapeutic target in AD. In illnesses like Alzheimer’s that cause brain deterioration, the brain’s defense cells, known as microglia, overreact due to harmful proteins, causing brain damage and memory loss. This research aimed to understand how microglia change in Alzheimer’s and find ways to stop their damaging effects. Using mice with Alzheimer’s, they checked if blocking specific immune pathway could fix microglia dysfunction and Alzheimer’s disease pathologies. They discovered that blocking STING, a crucial part of this pathway, reduced microglia dysfunction brain inflammation, decreased the buildup of Alzheimer’s-related proteins, and improved memory in mice. By blocking the STING activation, the study showed a decrease in damaging brain inflammation and improvements in memory function, suggesting a promising strategy for treating Alzheimer’s. Researchers conclude that targeting the STING could offer a new way to fight Alzheimer’s by reducing inflammation and protecting brain health. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
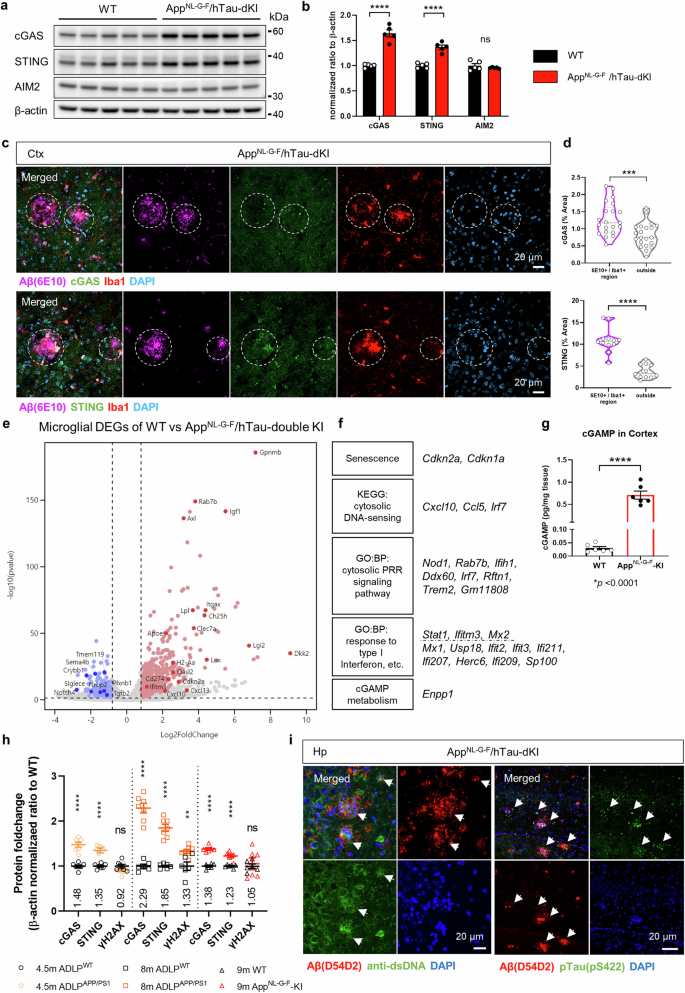
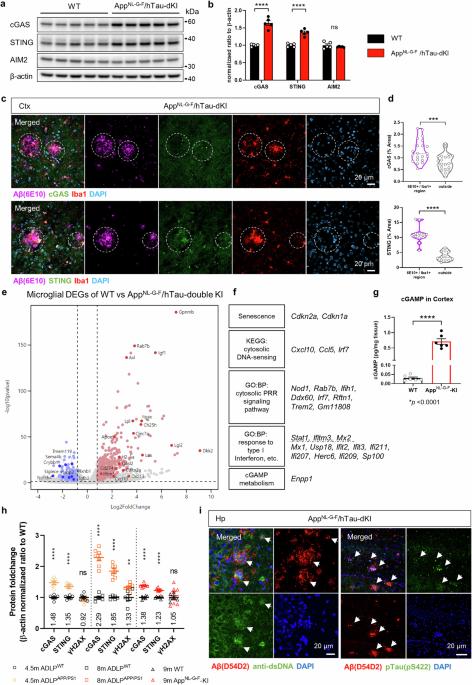
阻断 STING 激活可缓解小胶质细胞功能障碍和阿尔茨海默病的多种病理变化。
神经胶质的异常激活会促进阿尔茨海默病(AD)的神经变性,而阿尔茨海默病是导致痴呆症的最常见原因。cGAS-STING 通路的刺激会诱发小胶质细胞功能障碍和无菌性炎症,从而加剧阿尔茨海默病。我们的研究表明,抑制 STING 的激活可以控制小胶质细胞,并改善多种 AD 症状。cGAS-STING 通路是检测异位 DNA 和随后的免疫反应所必需的。淀粉样蛋白-β(Aβ)和tau诱导线粒体应激,导致DNA释放到小胶质细胞的胞浆中。作为AD风险因子的APOE ε4等位基因的存在也会上调这两种蛋白。STING 激活是小胶质细胞 NLRP3 激活、促炎反应和 I 型干扰素反应的必要条件。药理 STING 抑制可减少 AppNL-G-F/hTau 双基因敲入小鼠的多种 AD 致病特征。小胶质细胞的转录组发生了意想不到的变化,从而减少了胶质细胞增生和脑部炎症。Aβ负荷、tau磷酸化和小胶质细胞突触吞噬的显著减少防止了记忆丧失。总之,我们的研究描述了 STING 激活的致病机制及其作为 AD 治疗靶点的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


